
ij�о���ѧϰС��ͬѧ�������ͼ��ʾװ��̽�������Ļ�ԭ�ԡ�

(1)���������Ļ�ѧ����ʽΪ_____________________________________________
________________________________________________________________________��
(2)��ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ__________��
(3)��С��ͬѧ�����Ͷ�����̼Ϊԭ���Ʊ�̼����李���ͬѧ�Ƚ�������̼ͨ��ˮ�У�����ܽ����ͨ�백������ͬѧ�Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼����ѡ����ʵķ�����˵��ԭ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A����ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ
B���Ҵ������ᡢ�����������ܷ���ȡ����Ӧ
C�����ۡ���֬�������ʵ�ˮ����ﻥΪͬ���칹��
D����ά�ء�����ϩ�����ά�����ڸ߷��ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪(HF)2(g)2HF(g)����H>0����ƽ����ϵ��������(m��)�������ʵ���(n��)֮���ڲ�ͬ�¶�����ѹǿ�ı仯������ͼ��ʾ������˵����ȷ����(����)
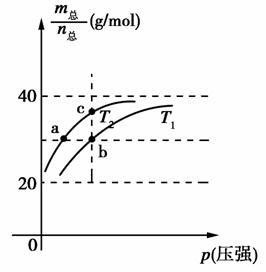
A���¶ȣ�T1<T2
B��ƽ�ⳣ����K(a)��K(b)<K(c)
C����Ӧ���ʣ�vb>va
D���� ��30 g/molʱ��n(HF):n[(HF)2]��2:1
��30 g/molʱ��n(HF):n[(HF)2]��2:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̬NH4Cl���ȱ�����壬���������ֱ�Ϊ��̬NH4Cl����̬�����ȱ�����������������ֱ�ɹ�̬�⣬����������ı����Ƿ���ͬ����˵���жϵ����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�ء���һ������������֮����ת����ϵ��ͼ��ʾ(���ַ�Ӧ�е�H2O����ȥ)��
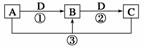
��ش��������⣺
(1)��A����������ˮ������D�����������������������;���Ľ������ʣ���������B����Һ���ܵõ�B����B�Ļ�ѧʽ������__________����ҵ����ȡA�����ӷ���ʽΪ______________________________________________________________________
________________________________________________________________________��
(2)��A��һ�ּ������壬�������������B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ________________________________________________________��
(3)��D���ȼҵ����Ҫ��Ʒ֮һ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ��________________________________________________________________________
______________________________________________________ __________________��
__________________��
(4)��A��C��D���dz������壬C�ǵ����������Ҫ���壬��Ӧ�۵Ļ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
ijͬѧ���Ѽ�����һ���������걣�����ܱ������У�ÿ��һ��ʱ��������pH����������ʼһ��ʱ���ڣ������pH�ʼ�С���ƣ������ӷ���ʽ����ԭ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� (����)
A������ֲ��ͨ�����������տ����еĵ��������ڻ�ѧ�仯
B������β�����ŷŵĵ�����������Ҫ��������̬��ת������
C����ʯȼ��ȼ��ͨ�����ͷų�����������
D��ֲ��ո�ȼ��ʱ�ų���������������˵���ѭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������NaOH��Һ����μ���AlCl3��Һ�����ɳ���Al(OH)3������AlCl3�������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ����������(����)
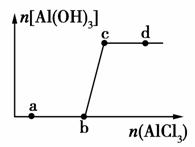
A��a���Ӧ����Һ�У�Na����Fe3����SO ��HCO
��HCO
B��b���Ӧ����Һ�У�Ag����Ca2����NO ��F��
��F��
C��c���Ӧ����Һ�У�Na����S2����SO ��Cl��
��Cl��
D��d���Ӧ����Һ�У�K����NH ��I����CO
��I����CO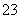
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E����ѧ������5�ֻ����A��B�������Ԫ��X��Y�ĵ����������г����� ������������ʼ�Ĺ�ϵ����ͼ��ʾ��
������������ʼ�Ĺ�ϵ����ͼ��ʾ��
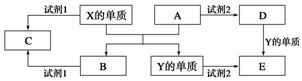
(1)X�ĵ�����A��Ӧ�Ļ�ѧ����ʽ��_______________________________________��
(2)���Լ�1��NaOH��Һ��X�ĵ������Լ�1��Ӧ�����ӷ���ʽ�� ________________________________________________________________
________________________________________________________________ ________��
________��
(3)���Լ�1���Լ�2����ϡ���ᡣ
�ټ�������D����Һ�н������ӵķ�����_____________________________________
________________________________________________________________________��
�ڽ�����C����ˮ������Һ�����ԣ�ԭ����(�����ӷ���ʽ��ʾ)________________________________________________________________________��
��ij��Ч��ˮ������Y(OH)SO4�ۺϵõ��ġ���ҵ����E��ϡ�������������Ϊԭ�����Ʊ�Y(OH)SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com