

���� ��1������ƿ��ʹ��ǰ�����©������ƿ�DZȽϾ��ܵ��������������ȣ��ʲ��������ܽ�����ϡ����Һ��Ҳ����������Ӧ�������ݴ˷�����
��2���������ʵ�����m=nM=cvM���㣻
��3���ܽ����ʱ�����Ǽ����ܽ⣬����ʱ���������ã�
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$������������Һ��Ũ��Ӱ�죮
��� �⣺��1��A��ʹ������ƿǰӦ�ü����Ƿ�©ˮ����A��ȷ��
B������ƿ��ˮϴ�������ô�����Һϴ�ӣ������Ӱ��������Һ��Ũ�ȣ���B����
C��������Һʱ����������ǹ��壬Ӧ�����ձ����ܽ⣬��ҩƷ��ȫ�ܽ�ָ������£��ٰ���ҺС�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߣ����ܰѳƺõĹ�����ֽ����������ƿ�У���C����
D��������Һʱ����������Һ�壬Ӧ�����ձ����ܽ⣬��ҩƷ��ȫ�ܽ�ָ������£��ٰ���ҺС�ĵ�������ƿ�У�������ˮ���ӽ��̶���1��2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߣ����ܰ�Һ��ҩƷֱ��������������ƿ�У���D����
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ���E��ȷ��
��ѡBCD��
��2��0.2mol•L-1Na2CO3��Һ250mL��ҪNa2CO3������Ϊ��0.25L��0.2mol/L��106g/mol=5.3g���ʴ�Ϊ��5.3��
��3�����������ܽ����ʱΪ�˼����ܽ⣬��������ã�����ʱ�����������ã�
�ʴ�Ϊ�����裻������
��4��A��ijͬѧ�ڵڢಽ�۲�Һ��ʱ���ӣ���Һ�����ƫ��������ҺŨ��ƫ�ڣ��ʴ�Ϊ��ƫ�ͣ�
B���ڲ�����У�ҩƷ�������̣�����������̣�ʹ�����룩������������ҩƷ������ƫС��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע���c=$\frac{n}{v}$������Һ����ԭ������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��м��ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2�� | |
| B�� | ϡ����������������Һ��Ӧ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O | |
| C�� | ������������ϡ���OH-+H+=H2O | |
| D�� | ������������������Ӧ��Ba2++SO42-=BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ƕ������ȷ�Ӧ | B�� | a��b��c��Ϊ��ֵ | ||
| C�� | a=b | D�� | 2a=c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ơ��ƿ�ǣ��ݳ��������� | |
| B�� | ʵ���ҿ������ű���ʳ��ˮ�ķ����ռ����� | |
| C�� | ��ͭ�ۺ�п�ۻ�Ϻ����ϡ�����У�������������ʱȲ���ͭ��ʱ�Ŀ� | |
| D�� | ��pH����3�Ĵ�����Һ�м�������CH3COONH4���壬��ҺpH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
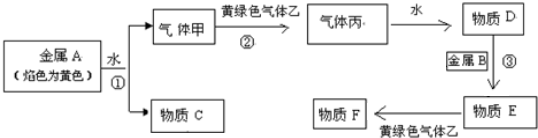
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com