
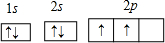
A��B��C���Ƕ�����Ԫ�أ�ԭ��������������B�ǵؿ��к�������Ԫ�أ�������AxBy�ж�����ʽ���еĿ��Ե������ꣻ������CmBnҲ�в�ͬ��ʽ������m��n������2��1��1��1�������ж���ȷ���ǣ�
A��ԭ�Ӱ뾶�ɴ�С��˳���ǣ�C>B>A
B��AxBy��A�Ļ��ϼ۲�����Ϊ+1��
C��C��B�γɵĻ���������ˮ���ܵõ�����B
D��A��B��Ԫ�ص���̬�⻯��������Ӧ
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


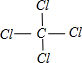
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

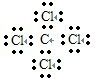
��֪C�ĺ˵��������A��B��Ԫ��ԭ�Ӻ��������֮�ͣ���д��A��BԪ���γɻ�����ĵ���ʽ��___________��д��B��CԪ�ؿ����γɵ����ֻ�����Ľṹʽ��___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com