
| ³ĮµķĪļ | Al£ØOH£©3 | Fe£ØOH£©3 | Fe£ØOH£©2 | Ni£ØOH£©2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
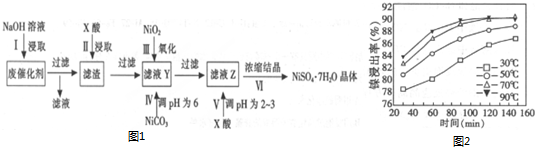
·ÖĪö ·Ļ“߻ƼĮÖĘČ”NiSO4•7H2O£¬ŗ¬Äų·Ļ“߻ƼĮÖ÷ŅŖŗ¬ÓŠNi£¬»¹ŗ¬ÓŠAl”¢FeµÄµ„ÖŹ¼°Ńõ»ÆĪļ£¬ĘäĖūŹĒ²»ČÜŌÓÖŹ£¬·Ļ“߻ƼĮÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ½žČ”£¬ĀĮ¼°ĘäŃõ»ÆĀĮČܽā£¬¹żĀĖµĆµ½ĀĖŌüĪŖÄų”¢Ģś¼°ĘäŃõ»ÆĪļ£¬¼ÓČėĮņĖįĖį½žÖ÷ŅŖŹĒČܽāÄų½šŹōŗĶĢśµ„ÖŹ¼°ĘäŃõ»ÆĪļ£¬¹żĀĖµĆµ½ĀĖŅŗÖŠ¼ÓČėNiO2Ńõ»ÆŃĒĢśĄė×ÓĪŖĢśĄė×Ó£¬NiCO3µ÷½ŚČÜŅŗPH=6³ĮµķĢśĄė×Ó£¬¹żĀĖµĆµ½ĀĖŅŗÖŠ¼ÓČėĮņĖįµ÷½ŚČÜŅŗPH=2-3£¬·ĄÖ¹ĮņĖįÄųČÜŅŗÖŠÄųĄė×ÓĖ®½āÉś³É³Įµķ£¬ÅØĖõ½į¾§µĆµ½NiSO4•7H2O£¬
£Ø1£©ŗ¬Äų“߻ƼĮÖ÷ŅŖŗ¬ÓŠNi£¬»¹ŗ¬ÓŠAl£Ø31%£©µÄµ„ÖŹ¼°Ńõ»ÆĪļ£¬ĀĮŗĶŃõ»ÆĀĮ¶¼æÉŅŌŗĶĒæĖįĒæ¼ī·“Ó¦ČܽāµĆµ½ČÜŅŗŗ¬ÓŠĘ«ĀĮĖįŃĪ£»
£Ø2£©øł¾ŻÄų½ž³öĀŹĖꏱ¼ä±ä»ÆĶ¼æÉÖŖ£¬70”ꏱ£¬Äų½ž³öĀŹŗÜ“ó£¬“ÓŹ±¼äæ“£¬120minÄų½ž³öĀŹ¾ĶŅŃ¾ŗÜøßĮĖ£»
£Ø3£©µ÷½ŚČÜŅŗpHĪŖĖįŠŌ·ĄÖ¹ÄųĄė×ÓĖ®½ā£¬¼õÉŁÄųĄė×ÓµÄĖšŹ§£»
£Ø4£©·ÅµēŹ±NiO£ØOH£©×Ŗ»ÆĪŖNi£ØOH£©2£¬NiŌŖĖŲ»ÆŗĻ¼Ū½µµĶ£¬±»»¹Ō£¬Ó¦ĪŖŌµē³ŲÕż¼«·“Ó¦£»
£Ø5£©ŅĄ¾ŻČČ»Æѧ·½³ĢŹ½ŗĶøĒĖ¹¶ØĀɼĘĖć¢Ū”Į2-¢Ś-¢ŁµĆµ½ŗ¬Äų“¢Ēā²ÄĮĻ£ØMg2NiH4£©ŹĶ·ÅĒāĘųŗĶMg2NiµÄČČ»Æѧ·½³ĢŹ½£»
£Ø6£©·ÖĪöæÉÖŖµŚ¢ņ²½¼ÓČėcmol/LµÄĮņĖįbL£¬ĪļÖŹµÄĮæĪŖcbmol£¬ŌŖĖŲŹŲŗć×īŗóĪŖNiSO4£¬ÄųŌŖĖŲĄ“Ō“ÓŚ¼ÓČėµÄNiCO3”¢NiO2ŗĶ·Ļ“߻ƼĮÖŠµÄNi£¬ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦ŗĶĢśµÄĮæ¼ĘĖćĻūŗÄNiO2ĪļÖŹµÄĮ棬ÄųŌŖĖŲŹŲŗć¼ĘĖćµĆµ½Ģ¼ĖįÄųĪļÖŹµÄĮæµĆµ½ÖŹĮ森
½ā“š ½ā£ŗ£Ø1£©¼ī½ž”±¹ż³ĢÖŠŹĒĪŖĮĖ³żČ„ĀĮ¼°ĘäŃõ»ÆĪļ£¬ĀĮŹĒĮ½ŠŌŌŖĖŲŗĶĒæ¼ī·“Ó¦£¬Ńõ»ÆĀĮŹĒĮ½ŠŌŃõ»ÆĪļŗĶĒæ¼ī·“Ó¦£¬Äųµ„ÖŹŗĶĢś¼°ĘäŃõ»ÆĪļ²»ŗĶ¼ī·“Ó¦“ļµ½³żČ„ĀĮŌŖĖŲµÄÄæµÄ£¬·“Ó¦µÄĮ½ÖÖ·½³ĢŹ½ĪŖ£ŗ2Al+2OH-+2H2OØT2AlO2-+3H2”ü”¢Al2O3+2OH-ØT2AlO2-+3H2O£¬µŚ1²½¼ÓČėNaOHČÜŅŗµÄÄæµÄŹĒ³żČ„Al”¢Al2O3ŗĶÓĶÖ¬µČŌÓÖŹ£¬
¹Ź“š°øĪŖ£ŗ³żČ„Al”¢Al2O3ŗĶÓĶÖ¬µČŌÓÖŹ£»
£Ø2£©”°Ėį½ž”±Ź±Ö÷ŅŖŹĒČܽāÄų½šŹōŗĶĢśµ„ÖŹ¼°ĘäŃõ»ÆĪļ£¬ŅĄ¾ŻÖʱøÄæµÄŹĒµĆµ½NiSO4•7H2O£¬¼ÓČėµÄĖį²»ÄÜŅżČėŠĀµÄŌÓÖŹ£¬ĖłŅŌŠčŅŖ¼ÓČėĮņĖį½ųŠŠĖį½žĖį½žXĪŖĮņĖį£¬øł¾ŻÄų½ž³öĀŹĖꏱ¼ä±ä»ÆĶ¼æÉÖŖ£¬70”ꏱ£¬Äų½ž³öĀŹŗÜ“ó£¬“ÓŹ±¼äæ“£¬120minÄų½ž³öĀŹ¾ĶŅŃ¾ŗÜøßĮĖ£¬
¹Ź“š°øĪŖ£ŗĮņĖį£»C£»
£Ø3£©ĮņĖįÄųČÜŅŗŠčŅŖÕō·¢ÅØĖõ½į¾§Īö³ö£¬ĪŖ·ĄÖ¹ÄųĄė×ÓĖ®½āÉś³ÉĒāŃõ»ÆÄų³Įµķ£¬ČÜŅŗÖŠ“ęŌŚĖ®½āĘ½ŗāNi2++2H2O?Ni£ØOH£©2+2H+£¬Ōö“óĒāĄė×ÓÅضČĘ½ŗāÄęĻņ½ųŠŠ±ÜĆāÅØĖõ¹ż³ĢÖŠĖ®½āÉś³É³Įµķ£¬ŠčŅŖæŲÖĘČÜŅŗpHŌŚĖįŠŌĢõ¼žĻĀ£¬
¹Ź“š°øĪŖ£ŗČÜŅŗÖŠ“ęŌŚĖ®½āĘ½ŗāNi2++2H2O?Ni£ØOH£©2+2H+£¬Ōö“óĒāĄė×ÓÅضČĘ½ŗāÄęĻņ½ųŠŠ±ÜĆāÅØĖõ¹ż³ĢÖŠĖ®½āÉś³É³Įµķ£»
£Ø4£©·ÅµēŹ±NiO£ØOH£©×Ŗ»ÆĪŖNi£ØOH£©2£¬NiŌŖĖŲ»ÆŗĻ¼Ū½µµĶ£¬±»»¹Ō£¬Ó¦ĪŖŌµē³ŲÕż¼«·“Ó¦£¬µē¼«·½³ĢŹ½ĪŖNiO£ØOH£©+H2O+e-ØTNi£ØOH£©2+OH-£¬
¹Ź“š°øĪŖ£ŗNiO£ØOH£©+H2O+e-ØTNi£ØOH£©2+OH-£»
£Ø5£©¢Ł2Mg£Øs£©+O2£Øg£©=2MgO£Øs£©”÷H1=-2075kJ/mol
¢ŚMg2Ni£Øs£©+2MgH2£Øs£©=2Mg£Øs£©+Mg2NiH4£Øs£©”÷H2=+84.6kJ/mol
¢ŪMgH2£Øs£©+$\frac{1}{2}$O2£Øg£©=MgO£Øs£©+H2£Øg£©”÷H3=-963kJ/mol£¬
ŅĄ¾ŻČČ»Æѧ·½³ĢŹ½ŗĶøĒĖ¹¶ØĀɼĘĖć¢Ū”Į2-¢Ś-¢ŁµĆµ½ŗ¬Äų“¢Ēā²ÄĮĻ£ØMg2NiH4£©ŹĶ·ÅĒāĘųŗĶMg2NiµÄČČ»Æѧ·½³ĢŹ½£ŗ
Mg2NiH4£Øs£©=Mg2Ni£Øs£©+2H2£Øg£©”÷H=+64.4KJ/mol
¹Ź“š°øĪŖ£ŗMg2NiH4£Øs£©=Mg2Ni£Øs£©+2H2£Øg£©”÷H=+64.4KJ/mol£»
£Ø6£©·ÖĪöæÉÖŖµŚ¢ņ²½¼ÓČėcmol/LµÄĮņĖįbL£¬ĪļÖŹµÄĮæĪŖcbmol£¬ŌŖĖŲŹŲŗć×īŗóĪŖNiSO4£¬ÄųŌŖĖŲĄ“Ō“ÓŚ¼ÓČėµÄNiCO3”¢NiO2ŗĶ·Ļ“߻ƼĮÖŠµÄNi£¬ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦ŗĶĢśµÄĮæ¼ĘĖćĻūŗÄNiO2ĪļÖŹµÄĮ棬Ėį½žŗóµÄĀĖŅŗY²»ŗ¬Fe3+£¬ĀĖŅŗZÖŠ²ŠĮōµÄĖįŗöĀŌ²»¼Ę£¬NiO2”«Ni2+”«2e-£¬Fe2+”«Fe3+”«e-£¬ŌņNiO2”«2Fe2+£¬n£ØFe£©=$\frac{a”Į1{0}^{3}”Į5.6%}{56}$=amol£¬NiO2ĪļÖŹµÄĮæĪŖ0.5amol£¬Ō·Ļ“߻ƼĮÖŠNiĪļÖŹµÄĮæ=$\frac{a”Į1{0}^{3}”Į29.5%}{59}$=5amol£¬ŌņNiCO3ĪļÖŹµÄĮæ=cbmol-0.5amol-5amol=£Øcb-5.5a£©mol£¬ŌņµŚ¢ō²½Ó¦¼ÓČėNiCO3 ÖŹĮæ=£Øcb-5.5a£©mol”Į119g/mol=119£Øcb-5.5a£©g=0.119£Øcb-5.5a£©Kg£¬
¹Ź“š°øĪŖ£ŗ0.119£Øcb-5.5a£©£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹ·ÖĄėĢį“攢³żŌÓŹµŃé¹ż³Ģ·ÖĪö”¢ŌŖĖŲŹŲŗćµÄ¼ĘĖć”¢øĒĖ¹¶ØĀɼĘĖćµČ£¬Ö÷ŅŖŹĒĄė×ÓŠŌÖŹŗĶĮ÷³Ģ·ÖĪöÅŠ¶Ļ£¬ÕĘĪÕ»ł“”ŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ɳ×ÓŗĶÕ³ĶĮÖ÷ŅŖ³É·ÖĪŖ¹čĖįŃĪ | |
| B£® | ”°Č¼Š½¾Ł»š”±Ź¹Õ³ĶĮ·¢Éśø“ŌÓµÄĪļĄķ»Æѧ±ä»Æ | |
| C£® | ÉÕÖĘŗó×ŌČ»ĄäČ“³ÉŗģĶߣ¬½½Ė®ĄäČ“³ÉĒąĶß | |
| D£® | Õ³ĶĮŹĒÖĘ×÷שĶßŗĶĢՓɵÄÖ÷ŅŖŌĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
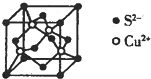 Įņ¼°Ęä»ÆŗĻĪļ¾ßÓŠ¹ć·ŗµÄÓĆĶ¾£®»Ų“šĻĀĮŠĪŹĢā£ŗ
Įņ¼°Ęä»ÆŗĻĪļ¾ßÓŠ¹ć·ŗµÄÓĆĶ¾£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 7 | B£® | 8 | C£® | 9 | D£® | 10 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
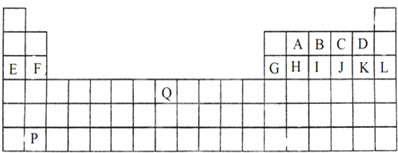
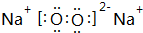 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com