
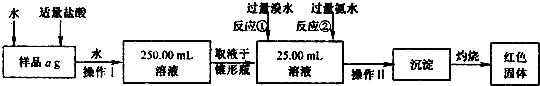
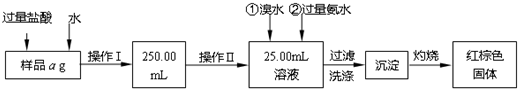
ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
��������������̣��ش��������⣺
��1������I���õ��IJ����������ձ����������⣬�������� �� �������������ƣ�
��2����д��������ˮ���������ӷ�Ӧ����ʽ ��
��3������������ȣ���ȴ�����£�����ƽ����������Ϊb1 g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1��b2��0.3g�����������Ӧ���еIJ�����
��
��������������W1 g������������Ⱥ������������W2 g������Ʒ����Ԫ�ص�����������
����ͬѧ����������Բ������·������ⶨ��
��1���ܽ���Ʒ���������ᣬ���������ᣬΪʲô
��2��ѡ��Ļ�ԭ���Ƿ������� ����ǡ�����ԭ���ǣ�
��3�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص�����������
��1��250mL����ƿ��1�֣�����ͷ�ιܣ�1�֣�
��2��2Fe 2+ �� Br2 �� 2Fe 3+ �� 2Br����2�֣�
��3���ٴμ�����ȴ��������ֱ������������С��0.1g��2�֣�
��Ԫ�ص�����������![]() ��3�֣�
��3�֣�
��1������������Ժ���KMnO4�ĵζ��и��ţ�1�֣�
��2����1�֣������������ԭ����������������ᷴӦ����Fe2+��������Ԫ�صIJⶨ��2�֣�
��3����Ԫ�ص�����������2.8bc/a��2�֣�
����:��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 700(W2-W1) |
| a |
| 700(W2-W1) |
| a |
| 280cd |
| b |
| 280cd |
| b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 1120(W2-W1) |
| 160a |
| 1120(W2-W1) |
| 160a |
| 14bc |
| 5a |
| 14bc |
| 5a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1120(W2-W1) |
| 160a |
| 1120(W2-W1) |
| 160a |
| 2.8bc |
| a |
| 2.8bc |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
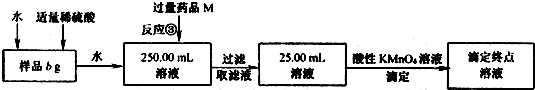
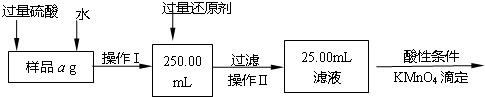
ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�

��������������̣��ش��������⣺
��1������I���õ��IJ����������ձ����������⣬�������� �� �������������ƣ�
��2����д��������ˮ���������ӷ�Ӧ����ʽ ��
��3������������ȣ���ȴ�����£�����ƽ����������Ϊb1 g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1��b2��0.3g�����������Ӧ���еIJ�����
��
��������������W1 g������������Ⱥ������������W2 g������Ʒ����Ԫ�ص�����������
����ͬѧ����������Բ������·������ⶨ��
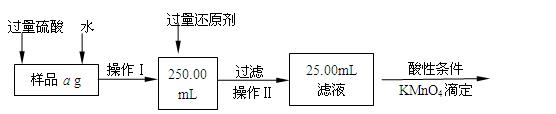
��1���ܽ���Ʒ���������ᣬ���������ᣬΪʲô
��2��ѡ��Ļ�ԭ���Ƿ������� ����ǡ�����ԭ���ǣ�
��3�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
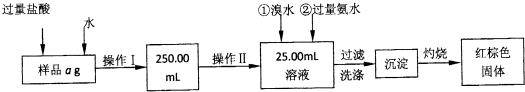
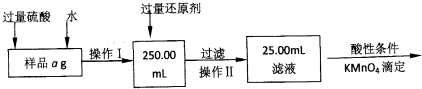
ij�Ȼ������Ȼ������Ļ�����Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
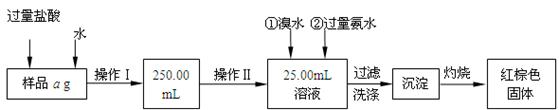
��������������̣��ش��������⣺
��1������I���õ��IJ����������ձ����������⣬�������� �� �������������ƣ�
��2����д��������ˮ���������ӷ�Ӧ����ʽ ��
��3������������ȣ���ȴ�����£�����ƽ����������Ϊb1 g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1��b2��0��3g�����������Ӧ���еIJ�����
��������������W1 g������������Ⱥ������������W2 g������Ʒ����Ԫ�ص�����
������
����ͬѧ����������Բ������·������ⶨ��
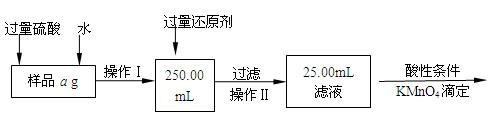
��1���ܽ���Ʒ���������ᣬ�����������ᣬΪʲô
��2��ѡ��Ļ�ԭ���Ƿ������� ����ǡ�����ԭ���ǣ�
��3�����ζ��õ�c mol/L KMnO4��ҺbmL������Ʒ����Ԫ�ص����������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com