
(13�֣������������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3,���Ʊ���Ӧ����ʽΪ��
o
(1) �����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����_______��
(2) �ø÷�����õ�Naj2O3^H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
��������衿
����1 ��������ֻ��>fe2C03����
����2:������ֻ��Na2S����
����3: ____________________________
���������ϡ�
��NhS2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��
���ж���˼����
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4,��������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������_______ (�����������������������˵�����ɣ�____________________________
����Ʒ�������ʵ�顿
���ڼ���1,����±�ʵ�鷽���������ۣ�������ѡ)��
��ѡʵ���Լ�������KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
(3) ��֪����Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
�ĵ�ˮ���ж��ȡ���ζ������
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
��1������ˮ�е��ܽ������������ ��2�֣�
��2�������к�Na2S��Na2CO3�������ʣ�2�֣�
����������1�֣� Na2S2O3��ϡH2SO4��Ӧ���ɵ�SO2��H2S������Ӧ��������H2S�ݳ�����2�֣�
| ʵ�鷽�� | ������ |
| ȡ������������ƿ�У���������3 mol∙L-1 H2SO4�����ϴ������ܵ���Ƥ���������������嵼��������ͨ��ʢ������KMnO4��Һ��Ʒ����Һ������ʯ��ˮ��ϴ��ƿ����2�֣� | Ʒ����Һ����ɫ������ʯ��ˮ����ǣ������к�Na2CO3���ʣ�2�֣� |
�����������𰸾����֣�
��3�������к������ʣ���Na2S�ȣ��ڵζ�ʱ���뷴Ӧ���岿��ʧȥ�ᾧˮ��2�֣������������𰸾����֣�
����:���������Na2S2O3��5H2O��ϡH2SO4��Ӧ���ɵ�SO2��H2S��CO2�ļ��鶼�и��ţ��ڼ���ʱӦ��ֿ��ǣ����ǵ������к��е����ʣ�Na2S���ڵζ�ʱ���뷴Ӧ���ǽ��ڣ�3��С��Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����1.520 g��Ʒ�м���̼�������Һ��0.13% I2���ȷ���Һ���ڷ�Һ©������15 min�����ӷ���ʽΪ��![]() +I2+2
+I2+2![]() ====
====![]() +2I-+2CO2��+H2O
+2I-+2CO2��+H2O
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250 mL��Һ��
���ڢ�������Һ��ȡ25 mL���μӼ��ᣬ��ȥ���й�����Br2��
����ʽΪ��Br2+HCOOH====2HBr+CO2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ��
���ñ��������������Һ�ζ�����������Һ��������0.1120 mol��L-1 Na2S2O3 15.10 mL�����ӷ���ʽΪ��I2+2![]() ====2I-+
====2I-+![]()
�ش��������⣺
(1)д���ڢ�������������������Ӧ�����ӷ���ʽ��
(2)����ΪʲôҪ��0.13% I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
(3)������Ʒ���������Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ����ʮУ�������ʽ������������ۺ��Ծ���ѧ���֣������棩 ���ͣ������
(13�֣������������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3,���Ʊ���Ӧ����ʽΪ��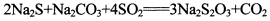 o
o
(1) �����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����_______��
(2) �ø÷�����õ�Naj2O3^H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
��������衿
����1 ��������ֻ��>fe2C03����
����2:������ֻ��Na2S����
����3: ____________________________
���������ϡ�
��NhS2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��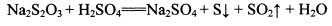
���ж���˼����
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4,��������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������_______ (�����������������������˵�����ɣ�____________________________
����Ʒ�������ʵ�顿
���ڼ���1,����±�ʵ�鷽���������ۣ�������ѡ)��
��ѡʵ���Լ���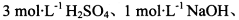 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
(3) ��֪�� ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������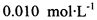 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������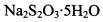 �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ����ʮУ�������ʽ������������ۺ��Ծ���ѧ���֣������棩 ���ͣ������
(13�֣������������һ�ֳ����Ļ���ԭ�ϡ���SO2ͨ�밴һ��������ɵ�Na2S��Na2CO3�Ļ����Һ�У���ɵõ�Na2S2O3,���Ʊ���Ӧ����ʽΪ��
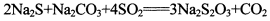 o
o
(1) �����ƻ����Һǰ�Ƚ�����ˮ�������һ��ʱ�����ã���Ŀ����_______��
(2) �ø÷�����õ�Naj2O3^H2O�����г�����һ���������ʡ�ij��ȤС�����������������ʳɷֽ���̽���������Ǹ���Ӧ�����������Ľᾧˮ����
����1 ��������ֻ��>fe2C03����
����2:������ֻ��Na2S����
����3: ____________________________
��NhS2O3�����ԡ�������Һ�н��ȶ�������������Һ����Ѹ�ٷ�Ӧ��
���ж���˼����
ijͬѧȡ�����Ƶõľ�����������ϡH2SO4,��������������ͨ��CuSO4��Һ�У�δ����ɫ�������ݴ���Ϊ����2������������Ϊ������Ƿ������_______ (�����������������������˵�����ɣ�____________________________
���ڼ���1,����±�ʵ�鷽���������ۣ�������ѡ)��
��ѡʵ���Լ��� ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ

(3) ��֪��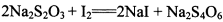 ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������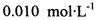 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������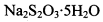 �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ʵ���Ҷ�������ij��Ʒ���������Ƶ�һ�ַ����ǣ�
����1.520g��Ʒ�м���̼�������Һ��0.13�� I2���ȷ���Һ���ڷ�Һ©������15min�����ӷ���ʽΪ��SO32����I2��2HCO3����SO42����2I����2CO2����H2O
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250mL��Һ��
���ڢ�������Һ��ȡ25mL���μӼ��ᣬ��ȥ���й�����Br2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ�����ӷ���ʽΪ��6H����IO3����5I����3I2��3H2O
���ñ��������������Һ�ζ�����������Һ��������0.1120mol/L Na2S2O3 15.10mL�����ӷ���ʽΪ��I2��252O32����2I����S4O62��
�ش��������⣺
��1��д���ڡ���������������������Ӧ�����ӷ���ʽ��
��2������ΪʲôҪ��0.13�� I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
��3��������Ʒ���������Ƶ������ٷֺ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com