
(11��)A��B��C��D��E������NH4Cl��Ba(OH)2��KCl��K2SO4��(NH4)2SO4��ɫ��Һ�е�һ�֣��������������ʱ�����������ǣ�
��A��B��Ϻ������ɫ���������Ⱥ�����������
��B��C���Ҳ������ɫ���������Ⱥ����������������ʹʪ��ĺ�ɫʯ����ֽ������
��B��E��Ϻ������������Ⱥ�Ҳ����ʹʪ��ĺ�ɫʯ����ֽ���������塣
��D���κ�һ����Һ��Ϻ������Ա仯�������������ش��������⣺
(1)A��___ _�� B�� ___��C��_____ ___��D��___ _____��E�ĵ���ʽ_____ __��
(2)д���йط�Ӧ�����ӷ���ʽ��
A��B��________________________________________________��
B��C��_________________________________________________��
B��E��_____________________________________________��
(1)K2SO4
Ba(OH)2 (NH4)2SO4
KCl 
��2��SO42- + Ba2+��BaSO4�� Ba2++ 2OH- + 2NH4+ + SO42-��BaSO4�� + 2NH3��+ 2H2O
NH4+ + OH-��NH3��+H2O
��������
������������ݢٿ�֪�����ɵİ�ɫ���������ᱵ���ҷ�Ӧ��û�а������ɣ�����ABӦ������������������ء����ݢڿ�֪�����ɵİ�ɫ���������ᱵ���ҷ�Ӧ���а������ɣ�����B������������C������泥���A��������ء����ݢۿ�֪�����ɵ������ǰ���������E���Ȼ�泥���D�����Ȼ��ء�
���㣺�������ʼ����Լ�����ʽ������ʽ����д��
�������������е��Ѷ�����Ŀ��飬�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ������������������ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶��������������������Ҫע������ڽ������ʵļ���ʱ��Ҫ�������ʵ��������ʺ�������Ӧ��ѡ���ʵ����Լ��ͷ�����ȷ�۲췴Ӧ�е�������������ɫ�ı仯�����������ɺ��ܽ⡢����IJ�������ζ���������ɫ�ȣ������жϡ���������֤���ɡ�


 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��)A��B��C��D��E��������ת����ϵ��ͼ��ʾ��

A�����ʣ� �ػ�ɫ����
��1��ȷ��A��B��C��D��E��Ϊʲô���ʣ���д�������ʵĻ�ѧʽ��
A ��B ��C ��D ��E ��
��2��д�����и�����Ӧ�Ļ�ѧ����ʽ��
E��A��
C��D��
E��C��
A��B��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ��һ�и���12���¿���ѧ�Ծ����������� ���ͣ������
(11��)A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��DԪ��ͬ���壬B��CԪ��ͬ���ڣ���A��B��C��D�е�����Ԫ�ؿ��γ�ԭ�Ӹ�����Ϊ1:1�Ķ��ֻ�����ס��ҡ�������Ϊ���е����֣����ǵ�Ԫ��������±���ʾ��
�����£�������Ϊ���壬�ܶ���С�ڿ�����������ΪҺ�壻�����ʺͶ�����Ϊ�����Ҷ�Ϊ���ӻ��������д���пհף�
��1�������ʵĵ���ʽΪ �����������������������ӵĸ���֮��Ϊ ��
��2������״����5.6L��������ȫȼ�շų�������ΪQKJ����д����ʾ������ȼ���ȵ��Ȼ�ѧ����ʽ ��
��3��B��C����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��2���γɻ������죬A��C��D����Ԫ�ذ�ԭ�Ӹ�����Ϊ1��1��1���γɻ����Z�������찴���ʵ���֮��Ϊ3:2��ȫ��Ӧ�����Һ�и�����Ũ�ȵĴ�С��ϵΪ ��
��4��ijͬѧ�����һ���Խṹ��ʽ��BA3��CA����Ϊȼ�ϵĵ�أ����øõ�ص��200mLһ��Ũ��NaCl��CuSO4�����Һ����װ������ͼ��
��д������ͨ���������һ���ĵ缫��Ӧʽ ��
�������Ϣ���������������������ʱ��仯�Ĺ�ϵ������ͼ��ʾ����������ѻ���ɱ�״���µ��������д����t1��ʯī�缫�ϵĵ缫��Ӧʽ ����t2ʱ������Һ��pHԼΪ ��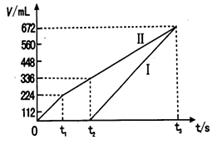
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����ͨ�ߵ�ѧУ����ͳһ���Ի�ѧ���⣨���Ͼ��� ���ͣ������
(11��)A��B��C��D1��D2��E��F��G��H��Ϊ�л���������������ͼʾ�ش����⡣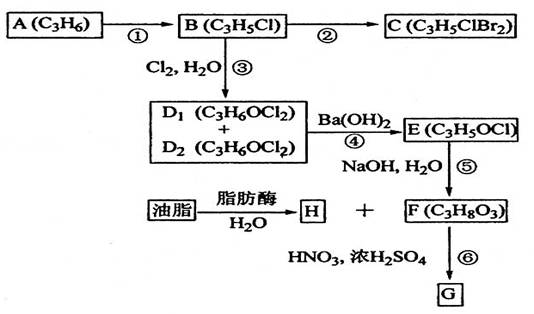
��1��ֱ���л�������A�Ľṹ��ʽ��_________________________��
��2���ٵķ�Ӧ�Լ��ͷ�Ӧ������____________________��
��3���۵ķ�Ӧ������_______________________________��
��4��B����C�Ļ�ѧ����ʽ��_______________________________��
D1��D2����E�Ļ�ѧ����ʽ��_____________________________��
��5��G��Ӧ����ҽ�ơ����Ƶȣ���F����G�Ļ�ѧ����ʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
(11��) A��B��C��D��E��F��G��H��I����ѧ��ѧ�г��������壬���Ǿ��ɶ�����Ԫ����ɣ������������ʣ�
��A��B��E��F��G��ʹʪ�����ɫʯ����ֽ��죬I��ʹʪ��ĺ�ɫʯ����ֽ������C��D��H����ʹʪ���ʯ����ֽ��ɫ��
��A��I����������ɫ���̣�
��B��E����ʹƷ����Һ��ɫ��
�ܽ����ȵ�ͭ˿����װ��B��ƿ�У�ƿ�ڳ����ػ�ɫ���̣�
�ݽ���ȼ��þ������װ��F��ƿ�У�þ������ȼ�գ����ɰ�ɫ��ĩ��ƿ�ڱڸ��ź�ɫ������
��C��D�������ɺ���ɫ���壻
��G��D��ȼ�տ��Բ���E��H2O��
�ཫB��H��ƿ�л�Ϻ����������ü����ӣ�ƿ�ڱڳ�����״Һ�β�����A��
�ش��������⣺
(1)A�Ļ�ѧʽ��________�������̵Ļ�ѧʽ��________��
(2)���з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(3)���з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)C�Ļ�ѧʽ��__________��D�Ļ�ѧʽ��__________��
(5)���з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(6)H�Ļ�ѧʽ��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com