
(1)�ڱ�״���£�a.6.72 L CH4���塡b��3.01��1023��HCl������ӡ�c��13.6g H2S���塡d��0.2mol NH3�����ж�����������Ĺ�ϵ�Ӵ�С��������(��������ű�ʾ)��
��������������ʵ���___________________________________��
�ڱ�״��������������ܶ�_____________________________��
���������������_______________________________________��
(2)�����ӵ�������������һ��ʵ�飺һ��յ��ܱ�������Mg���ڱ�״���£�ʢ������ͬ���ʵ�����ϵ�NO��H2�Ļ���������Ϊ(M��Q)g���ѻ�������ž����ٳ���SO2���壬Ϊʹ��ƽƽ�⣬Ӧ��������ƽ��________�������Ϸ���________g���롣
(3)��10g����CuSO4��x(NH4)2SO4��yH2O�ӵ�������NaOH��Һ�м��ȣ����ɵİ�����100 mL 0.5mol/L����ȫ�����գ������������2mol/L NaOH��Һ�кͣ���ȥNaOH��Һ25 mL����֪������SO����������Ϊ48%����x��________��y��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���������������������Ӧ����0.25 g�����ĵ�314 mL����(��״��)���������PCl3��PCl5�����ʵ���֮�Ƚӽ���(�� ��)
A�� 3��1������������B��5��3 C.2��3 D��1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ����۵�282 �桢�е�315 �棬�����տ������ˮ�ֶ����⡣���ɹ㷺������ˮ���������ӹ�ҵ��ӡȾҵ������ҵ��ij��ѧʵ��С���ͬѧ�������������Ʊ�FeCl3���塣
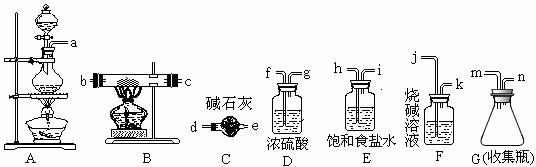
(1)д���Ʊ�����ʱ�����ӷ���ʽ_______________________
(2)��ѡ�õ���������˳��(��д�ӿ���ĸ)Ϊ___________n��d__________
(3)ʵ������У���A��B�������ȵ�˳��Ϊ_____________________������������Ŀ����_______________������Ϊ��ȼB���ƾ��Ƶ�ʵ���־��_________________����C������D���棬��ʹ��C��Ŀ����______________________����ȱ�ٴ���װ�ã���B�п��ܷ�������һ����Ӧ�ķ���ʽΪ________________________��
(4)��ͬѧ��Ϊ������ʹ��Eװ�ã���B�л���FeCl2���ɣ������һ��ʵ��ȷ���˹۵��Ƿ���ȷ________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A��1 Lˮ���ܽ���58.5 g NaCl������Һ�����ʵ���Ũ��Ϊ1 mol/L
B����1 L 2 mol/L��H2SO4��Һ��ȡ��0.5 L������Һ��Ũ��Ϊ1 mol/L
C������500 mL 0.5 mol/L��CuSO4��Һ����62.5 g����
D���к�100 mL 1 mol/L��H2SO4��Һ����NaOH 4 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʵ�����Ϊa mol��Na��AlһͬͶ��m g����ˮ�У�������Һ���ܶ�Ϊ�� g��cm��3�������Һ�����ʵ���Ũ��Ϊ
A��mol��L��1�� B��mol��L��1
C��mol��L��1 D��mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����
A��25�棬pH��2��HCl��Һ�к���H������ĿΪ0.01NA
B��1mol Na2O2�����к��е�������������Ϊ4 NA
C�����³�ѹ�£�14g��ϩ�ͻ�����Ļ�����У�����̼ԭ�ӵ���ĿΪNA
D����״���£�2.24LCl2��������NaOH��Һ��Ӧ��ת�Ƶĵ�����ĿΪ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����漰�л�������ķ����ᴿ��˵����ȷ����
A����ȥ�����л��е���ϩ���ɽ��������ͨ�����Ը��������Һ
B�����顢��ϩ�ͱ��ڹ�ҵ�϶���ͨ��ʯ�ͷ���õ�
C����ȥ�屽�л��е�Br2������NaOH��Һϴ�ӷ�Һ
D�����������е�����������Ҵ���������������Һ��ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���Ի�����Ϊԭ���������ᣬ������Ҫ��һ���Ǵ�����(�����б��ֺ��º�������)��2SO2(g)��O2(g)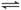 2SO3(g) ��H����196.6 kJ��mol��1
2SO3(g) ��H����196.6 kJ��mol��1
(1)������Ϊ��߷�Ӧ���ʺ�SO2��ת���ʣ����д�ʩ������ ��
A����װ���г���O2 B�������¶�
C����װ���г���N2 D����װ���г��������SO2
(2)���º�ѹ��ͨ��3mol SO2 ��2mol O2 �����������ƽ��ʱ�������������Ϊ��ʼʱ��90%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼ���ʵ�����Ϊ 5mol SO2(g)��3.5 mol O2(g)��1mol SO3(g)������˵����ȷ����
 A����һ��ƽ��ʱ��Ӧ�ų�������Ϊ294.9kJ
A����һ��ƽ��ʱ��Ӧ�ų�������Ϊ294.9kJ
B������ƽ��SO2��ת�������
C������ƽ��ʱ��O2����������
D���ڶ���ƽ��ʱSO3�������������2/9
(3)500 ��ʱ��10 mol SO2��5.0 mol O2�������Ϊ1�̵ĺ����ܱ������У�SO2ת��ΪSO3��ƽ��ת����Ϊ0.95����500��ʱ��ƽ�ⳣ��K= ��
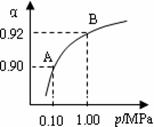
(4)550 �棬A��B��ʾ��ͬѹǿ�µ�ƽ��ת����(��ͼ)��
ͨ����ҵ�����в��ó�ѹ��ԭ���� ��
���Ƚϲ�ͬѹǿ�µ�ƽ�ⳣ����K(0.10 MPa) K(1.0 MPa)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���г�ȥ���ʵķ�����ȷ���ǣ� ��
| ���� | ���� | �Լ� | ��Ҫ���� | |
| A | NaHCO3���� | Na2CO3���� | / | ���� |
| B | SiO2 | Fe2O3 | ���� | ���� |
| C | KBr��Һ | Br2 | KOH��Һ | ��Һ |
| D | Cl2 | HCl | ����̼������Һ | ϴ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com