
(13·Ö)ŅŃÖŖ£ŗÕżŃĪAĒæČČæɵƵ½B”¢C”¢D”¢EĖÄÖÖĪļÖŹ£¬BĶس£ĒéæöĻĀĪŖĪŽÉ«ĪŽĪ¶ŅŗĢ壬E”¢F ŹĒæÕĘųÖ÷ŅŖ³É·Ö£¬DÄܲśÉśĖįÓź£¬IĪŖŗģ×ŲÉ«ĘųĢ壬CÓėJ·“Ó¦æɵĆA£¬J”¢KĪŖĮ½ÖÖ³£¼ūµÄĖį”£ĪļÖŹÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾(Ķ¼ÖŠ²æ·Ö·“Ó¦Īļ»ņÉś³ÉĪļ¼°·“Ó¦Ģõ¼žĪ“ĮŠ³ö£©”£ 
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)EĪļÖŹµÄµē×ÓŹ½ŹĒ________”£
(2)¼ģŃéCµÄŹŌÖ½ŹĒ________£¬¼ģŃéDµÄŹŌ¼ĮŹĒ________(ĢīŹŌÖ½”¢ŹŌ¼ĮĆū³Ę)”£
(3)Š“³öAĒæČČ·Ö½āÉś³ÉB”¢C”¢D”¢EµÄ»Æѧ·½³ĢŹ½________”£
(4)Š“³öDĶØČĖFeCl3ČÜŅŗŹ±£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½_____ ”£
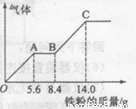
(5) ”Ŗ¶ØÅضČJ”¢K»ģŗĻŗóµÄĻ”ČÜŅŗ200mL£¬Ę½¾ł·Ö³ÉĮ½·Ż”£ĻņĘäÖŠŅ»·ŻÖŠÖš½„¼ÓČĖĶ·Ū£¬×ī¶ąÄÜČܽāa g(²śÉśĘųĢåÖ»ĪŖG)”£ĻņĮķŅ»·ŻÖŠÖš½„¼ÓČĖĢś·Ū£¬²śÉśĘųĢåµÄĮæĖęĢś·ŪÖŹĮæŌö¼ÓµÄ±ä»ÆČēĶ¼ĖłŹ¾”£Ōņ¢Ła£½________g£¬¢ŚĘųĢåG±ź×¼×“æöĻĀĢå»żĪŖ________£¬¢ŪJµÄĪļÖŹµÄĮæÅضČĪŖ______”£
£Ø13·Ö£©£Ø1£© £Ø2·Ö£© £Ø2£©ŗģÉ«ŹÆČļŹŌÖ½ Ę·ŗģČÜŅŗ £Ø2·Ö£©
£Ø2·Ö£© £Ø2£©ŗģÉ«ŹÆČļŹŌÖ½ Ę·ŗģČÜŅŗ £Ø2·Ö£©
£Ø3£©3(NH4)2SO4 6H2O£«4NH3”ü£«3SO2”ü£«N2”ü £Ø3·Ö£©
6H2O£«4NH3”ü£«3SO2”ü£«N2”ü £Ø3·Ö£©
£Ø4£©SO2£«2Fe3£«£«2H2O£½SO42££«2Fe2£«£«4H£« £Ø3·Ö£©
£Ø5£©¢Ł9.6g £Ø1·Ö£© ¢Ś2.24L £Ø1·Ö£© ¢Ū2.5mol/L £Ø1·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗBĶس£ĒéæöĻĀĪŖĪŽÉ«ĪŽĪ¶ŅŗĢ壬Ņņ“ĖBÓ¦øĆŹĒĖ®”£E”¢F ŹĒæÕĘųÖ÷ŅŖ³É·Ö£¬Ōņ¶žÕߏĒµŖĘųŗĶŃõĘų”£DÄܲśÉśĖįÓź£¬ĖłŅŌDŹĒSO2”£SO2ÄÜŗĶF·“Ӧɜ³ÉH£¬ŌņFŹĒŃõĘų£¬EŹĒµŖĘų£¬HŹĒČżŃõ»ÆĮņ”£ČżŃõ»ÆĮņŗĶĖ®·“Ӧɜ³ÉJ£¬JŹĒĮņĖį”£IĪŖŗģ×ŲÉ«ĘųĢ壬ŌņIŹĒNO2”£GŗĶŃõĘų·“Ӧɜ³ÉNO2£¬ĖłŅŌGŹĒNO”£NO2ČÜÓŚĖ®Éś³ÉĻõĖįŗĶNO”£KŹĒĖį£¬ŌņKŹĒĻõĖį”£CŗĶŃõĘų·“Ӧɜ³ÉNO£¬ĒŅCÓėJ·“Ó¦æɵĆA£¬ĖłŅŌCŹĒ°±Ęų£¬A¾ĶŹĒĮņĖįļ§”£
£Ø1£©µŖĘųŹĒŗ¬ÓŠ·Ē¼«ŠŌ¼üµÄµ„ÖŹ£¬Ęäµē×ÓŹ½ŹĒ ”£
ӣ
£Ø2£©°±ĘųŹĒ¼īŠŌĘųĢ壬æÉÓĆŗģÉ«ŹÆČļŹŌÖ½¼ģŃ飻SO2¾ßÓŠĘư׊Ō£¬æÉÓĆĘ·ŗģČÜŅŗ¼ģŃ锣
£Ø3£©AĒæČČ·Ö½āÉś³ÉB”¢C”¢D”¢EµÄ»Æѧ·½³ĢŹ½3(NH4)2SO4 6H2O£«4NH3”ü£«3SO2”ü£«N2”ü”£
6H2O£«4NH3”ü£«3SO2”ü£«N2”ü”£
£Ø4£©SO2¾ßÓŠ»¹ŌŠŌ£¬Äܱ»ĢśĄė×ÓŃõ»Æ£¬Ņņ“ĖøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒSO2£«2Fe3£«£«2H2O£½SO42££«2Fe2£«£«4H£«”£
£Ø5£©ĻõĖįŹĒŃõ»ÆŠŌĖį£¬ĖłŅŌøł¾ŻĶ¼ĻńæÉÖŖ£¬O”śA·¢ÉśµÄ·“Ó¦ŹĒFe£«4H£«£«NO3££½Fe3£«£«NO”ü£«2H2O”£A”śB·¢Éś·“Ó¦µÄ·½³ĢŹ½ŹĒ2Fe3£«£«Fe£½3Fe2£«£»B”śC·¢Éś·“Ó¦µÄ·½³ĢŹ½ŹĒFe£«2H£«£½Fe2£«£«H2”ü”£Čż½×¶ĪÖŠĻūŗÄĢśµÄĪļÖŹµÄĮæ·Ö±šŹĒ5.6g”Ā56g/mol£½0.1mol”¢£Ø8.4g£5.6g£©”Ā56g/mol£½0.05mol”¢£Ø14.0g£8.4g£©”Ā56g/mol£½0.1mol£¬ĖłŅŌøł¾ŻµŖŌŖĖŲŹŲŗćæÉÖŖ£¬ĻõĖįµÄĪļÖŹµÄĮæŹĒ0.1mol”£×īÖÕČÜŅŗÖŠÖ»ÓŠĮņĖįŃĒĢś£¬Ōņøł¾ŻŌ×ÓŹŲŗćæÉÖŖ£¬ĮņĖįŃĒĢśµÄĪļÖŹµÄĮæŹĒ0.25mol£¬Ņņ“ĖĮņĖįµÄĪļÖŹµÄĮæŅ²ŹĒ0.25mol£¬ŌņĮņĖįµÄÅØ¶ČŹĒ0.25mol”Ā0.1L£½2.5mol/L”£ĮķŅ»·ŻČÜŅŗÖŠ£¬ĒāĄė×ÓŗĶNO3£µÄĪļÖŹµÄĮæ·Ö±šŹĒ0.6molŗĶ0.1mol£¬Ōņøł¾Ż·½³ĢŹ½3Cu£«8H£«£«2NO3££½3Cu2£«£«2NO”ü£«4H2OæÉÖŖ£¬ĒāĄė×Ó¹żĮ棬ĖłŅŌNO3£ĶźČ«±»»¹ŌÉś³ÉNO£¬ŌņNOµÄĪļÖŹµÄĮæŹĒ0.1mol£¬ŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ2.24L”£ĘäÖŠČܽāĶµÄÖŹĮæŹĒ0.15mol”Į64g/mol£½9.6g”£
æ¼µć£ŗæ¼²éĪļÖŹĶʶĻ£»ĘųĢå¼ģŃ飻ĻõĖįŗĶĮņĖįµÄ»ģŗĻŅŗÓė½šŹō·“Ó¦µÄ¼ĘĖćµČ


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com