
ʵ������Ҫ����0.5mol?L-1���ռ���Һ500mL��������Һ���ƵĹ��̣��ش��������⣺
��1��ʵ���г���������ƽ�������룩��ҩ�ס���Ͳ�Ͳ������⣬����Ҫ���������������У� ��
��2�����ݼ����֪������NaOH���������Ϊ g��
��3��������Һ�Ĺ����У������²��������У���ȷ���� ������ţ���
A�����������ƹ������ֽƬ�ϳ�����
B�����ձ����ܽ��������ƹ������������Һ��������ƿ�У�
C�����ܽ��������Ƶ��ձ���������ˮϴ��2�D3�Σ�����ϴ��Һת�Ƶ�����ƿ�С�
��4���������ڸ�ʵ���е������У���
��
��
��
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�γ���ѧ2008��2009ѧ��ȸ߶���ѧ�����п���(��ѧ)(����) ���ͣ�013
|
ʵ����Ҫ����0.5 mol/L������Һ500 mL����Ҫ��10 mol/L��������Һ������� | |
| [����] | |
A�� |
25 |
B�� |
25 L |
C�� |
25 mL |
D�� |
50 mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����

�ڳ���������NaCl����Ӧ������ƽ��________(����̡������̡�)��
�۳�����ϣ���ҩƷ�����ձ��С�
(4)�ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����________________________��
(5)ת�ơ�ϴ�ӡ���ת��ʱӦʹ��________��������Ҫϴ���ձ�2��3����Ϊ��_________________�����ݣ�ҡ�ȡ�����õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ��
(6)�����ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�__________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
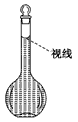
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����������0. 5 mol��L��1��ϡH2SO4500 mL��
��1����Ҫ98%��ŨH2SO4����=1.84/cm3�� mL��
��2����Ҫ�õIJ����������ձ�������������Ͳ�⣬���� ��
��3��ijͬѧ��������Һʱ�����������������������Һ�����ʵ���Ũ���к�Ӱ�죿����ƫ�ߡ�ƫ�ͻ䣩
����Һʱδ�ò���������ʹ����Һ������ ��
�ڶ��ݸ��ӿ̶��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ����0.50 mol/L NaCl��Һ480 mL�������в������������ʵ������֣���ʹ��������������
(1)ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������________��________�Լ��������ļ�Ƭ��ֽ��
(2)���㡣���Ƹ���Һ��ȡNaCl���������Ϊ________g��
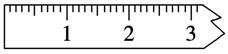 (3)������
(3)������
����ƽ��ƽ֮��Ӧ����ƽ���������ij��λ�ã�������ͼ����һ�����߱���������Ե������λ�ã�
�ڳ���������NaCl����Ӧ������ƽ��________(����̡������̡�)��
�۳�����ϣ���ҩƷ�����ձ��С�
(4)�ܽ⡣�ò�ʵ������Ҫʹ�ò�������Ŀ����_______________________________��
(5)ת�ơ�ϴ�ӡ���ת��ʱӦʹ��________������ϴ���ձ�2��3����____________________��
(6)���ݣ�ҡ�ȡ�
(7)��������Һ������ϡ��Ũ��Һ���Ĺ����У�������������һЩ����������������������Һ��Ũ�Ȼ��ܵ�Ӱ�죬������±����ڿո���ƫ��ƫС������Ӱ�죩��
| ��������һЩ������� | ��CB��Ӱ�� |
| ����ʱ�����ӿ̶��� | |
| ת����Һʱ���������¶˿�������ƿ�̶������� | |
| ϴ����ȡŨ��Һ����Ͳ����ϴ��Һת�Ƶ�����ƿ | |
| ���ݺ���ҡ�ȡ����á�Һ����ڿ̶����ټ�ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com