
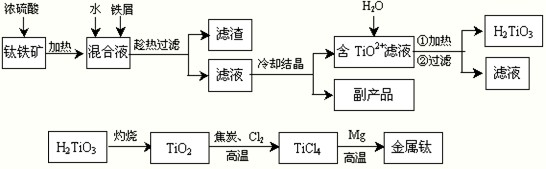
 H2TiO3+2H+
H2TiO3+2H+ H2TiO3+2H+
H2TiO3+2H+
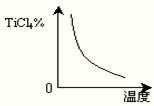
| ||
| c(TiCl4)��c2(CO) |
| c2(Cl2) |
| c(TiCl4)��c2(CO) |
| c2(Cl2) |
 H2TiO3+2H+��
H2TiO3+2H+�� H2TiO3+2H+���ٽ�TiO2+��ˮ�⣻
H2TiO3+2H+���ٽ�TiO2+��ˮ�⣻| c(TiCl4)��c2(CO) |
| c2(Cl2) |
| c(TiCl4)��c2(CO) |
| c2(Cl2) |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
�й�����������Ȼ��Դ�ḻ���ڿ����Դ�У��ѡ�ͭ������Ǧ��п��30���ֿ��������ȫ����һ����֦���ķ����������ϵ��������ȫ����ռʮ����Ҫ�ĵ�λ��
(1)�����������Ҫ�ɷֿ��û�ѧʽ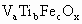 ����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��
����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��
(2)�����뷯������ʯ���տɵ�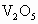 ��
��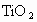 �����ʣ���д��
�����ʣ���д��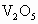 ����Al�۷�Ӧ�Ļ�ѧ����ʽ_____________��
����Al�۷�Ӧ�Ļ�ѧ����ʽ_____________��
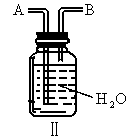
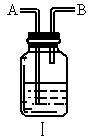
(3)��֪ ��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�
��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ� ��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��
��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��
(4)װ��ͼ��������ռ����������е�_________________(����ĸ)��
|
A��CO |
B��HCl |
C�� |
|
D�� |
E�� |
F�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
�й�����������Ȼ��Դ�ḻ���ڿ����Դ�У��ѡ�ͭ������Ǧ��п��30���ֿ��������ȫ����һ����֦���ķ����������ϵ��������ȫ����ռʮ����Ҫ�ĵ�λ��
(1)�����������Ҫ�ɷֿ��û�ѧʽ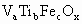 ����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��
����ʾ������V������5�ۣ�Ti�ԣ�4�ۣ�Fe�ԣ��ۣ���x��ֵ��_______(�ú�a��b��c�Ĵ���ʽ��ʾ)��
(2)�����뷯������ʯ���տɵ�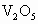 ��
��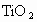 �����ʣ���д��
�����ʣ���д��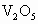 ����Al�۷�Ӧ�Ļ�ѧ����ʽ_____________��
����Al�۷�Ӧ�Ļ�ѧ����ʽ_____________��
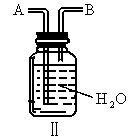
(3)��֪ ��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�
��ijѧ����ȤС�����һ��ʵ��װ������������Ӧ���Ƶ�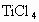 ��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��
��װ����β������������ͼ����ʾ��ƿ��Ӧ����_____________��Һ���ڵ��ܿ�B��Ӧ______________��
(4)װ��ͼ��������ռ����������е�_________________(����ĸ)��
|
A��CO |
B��HCl |
C�� |
|
D�� |
E�� |
F�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com