
ЁОЬтФПЁПвдМзШЉКЭБНЗгЮЊжївЊдСЯЃЌОЯТСазЊЛЏПЩКЯГЩЗгШЉЪїжЌКЭживЊЕФгаЛњКЯГЩжаМфЬхD(ВПЗжЗДгІЬѕМўКЭВњЮявбТдШЅ)

вбжЊЃКR1CH2COOCH2+R2COOCH3![]() CH3OH+
CH3OH+
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)ЗДгІЂйЕФЗДгІРраЭЮЊ___________ЃЛAЕФЛЏбЇУћГЦЮЊ___________ЁЃ
(2)CжаЫљКЌЙйФмЭХЕФУћГЦЮЊ___________ЃЛDЕФЗжзгЪНЮЊ___________ЁЃ
(3)ЗДгІЂкЕФЛЏбЇЗНГЬЪНЮЊ______________________ЁЃ
(4)AЕФЯТСааджЪжаЃЌФмЗДгГжЇСДЖдБНЛЗНсЙЙВњЩњгАЯьЕФЪЧ___________(ЬюзжФИ)ЁЃ
aЃЎФмгыЧтбѕЛЏФЦШмвКЗДгІ
bЃЎФмЪЙЫсадИпУЬЫсМиШмвКЭЪЩЋ
cЃЎЯђЯЁШмвКжаМгШыХЈфхЫЎКѓЃЌВњЩњГСЕэ
(5)ЗМЯуЛЏКЯЮяE(C8H10O2)гыBЕФЫЎНтВњЮяЛЅЮЊЭЌЗжвьЙЙЬхЃЌ1molEПЩгы2 mol NaOHЗДгІЃЌЦфКЫДХЙВеёЧтЦзжага3зщЗхЧвЗхУцЛ§жЎБШЮЊ3ЃК1ЃК1ЃЌдђEга___________жжПЩФмЕФНсЙЙ(ВЛПМТЧСЂЬхвьЙЙ)ЁЃ
(6)вдCH3OHКЭCH3ONaЮЊдСЯ(ЦфЫћЪдМСШЮбЁ)ЃЌЩшМЦжЦБИCH3COCH2 COOCH3ЕФКЯГЩТЗЯпЃКCH3OH![]() ______ЁЃ
______ЁЃ
ЁОД№АИЁПМгГЩЗДгІ СкєЧМзЛљБНЗг УбМќКЭѕЅЛљ C19H20O5 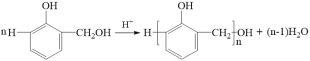 c 4 CH3OH
c 4 CH3OH![]() CH3Cl
CH3Cl![]() CH3CN
CH3CN![]() CH3COOCH3
CH3COOCH3![]() CH3COCH2COOCH3
CH3COCH2COOCH3
ЁОНтЮіЁП
ЃЈ1ЃЉЗДгІЂйжИдкЫсДпЛЏЯТЃЌЕШЮяжЪЕФСПЕФБНЗггыМзШЉЗДгІЃЌБНЗгСкЖдЮЛЕФЧтдзггыМзШЉЕФєЪЛљМгГЩЩњГЩєЧМзЛљБНЗгЃЌвђЖјЗДгІРраЭЪЧМгГЩЗДгІЃЌВњЮяAЕФУћГЦЪЧСкєЧМзЛљБНЗгЁЃ
ЃЈ2ЃЉCЪЧЖдМзбѕЛљБНввЫсМзѕЅЃЌЦфНсЙЙМђЪНЮЊ![]() ЃЌПЩжЊCКЌгаУбМќКЭѕЅЛљЃЌDЗжзгЪНЮЊC19H20O5ЁЃ
ЃЌПЩжЊCКЌгаУбМќКЭѕЅЛљЃЌDЗжзгЪНЮЊC19H20O5ЁЃ
ЃЈ3ЃЉЗДгІЂкЪЧСкєЧМзЛљБНЗгжЎМфЯрЛЅЭбЫЎЫѕКЯГЩЯпаЭНсЙЙИпЗжзгЃЌЦфЛЏбЇЗНГЬЪНЃК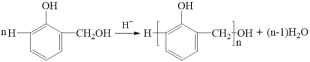 ЁЃ
ЁЃ
ЃЈ4ЃЉAЪЧБНЗгЃЌВрСДєЧЛљЛсЛюЛЏБНЛЗЧтдзгЃЌЪЙЕУвзЗЂЩњСкЮЛКЭЖдЮЛЕФШЁДњЗДгІЃЌР§ШчБНЗгКЭфхЫЎЗДгІЩњГЩ2,4,6-Ш§фхБНЗгЃЌЙЪД№АИбЁcЁЃ
ЃЈ5ЃЉИљОнEЕФЗжзгЪНC8H10O2ПЩЫуГіВЛБЅКЭЖШЮЊ4ЃЌНсКЯEЪЧЗМЯузхЛЏКЯЮяЃЌЫЕУїКЌгаБНЛЗЃЌВрСДЮоВЛБЅКЭМќЛђепЛЗЃЌгж1molEПЩгы2 mol NaOHЗДгІЃЌЫЕУїБНЛЗСЌзХСНИієЧЛљЃЌзюКѓЦфКЫДХЙВеёЧтЦзжага3зщЗхЧвЗхУцЛ§жЎБШЮЊ3ЃК1ЃК1ЃЌЕїећВрСДСНИіЬМдзгКЭєЧЛљЕФЮЛжУЃЌЗћКЯЬѕМўЙВга4жжЃЌЗжБ№ЪЧ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ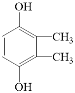 КЭ
КЭ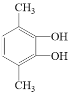 ЁЃ
ЁЃ
ЃЈ6ЃЉНсКЯЬтЩшПђЭМКЯГЩDЕФЗДгІТЗОЖЃЌЙиМќвЊКЯГЩввЫсМзѕЅCH3COOCH3ЃЌРћгУаХЯЂжаЬсЙЉЕФЗДгІБуПЩжЦБИCH3COCH2 COOCH3ЃЌвђЖјТЗЯпЩшжУЮЊ![]()
![]() ЁЃ
ЁЃ


 ПкЫуЬтПЈББОЉИОХЎЖљЭЏГіАцЩчЯЕСаД№АИ
ПкЫуЬтПЈББОЉИОХЎЖљЭЏГіАцЩчЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЪЕбщаЁзщЖдFeCl3ЗжБ№гыNa2SO3ЁЂNaHSO3ЕФЗДгІНјааЬНОПЁЃ
ЃЈМзЭЌбЇЕФЪЕбщЃЉ
зАжУ | БрКХ | ЪдМСX | ЪЕбщЯжЯѓ |
| I | Na2SO3ШмвК(pHЁж9) | БеКЯПЊЙиКѓСщУєЕчСїМЦжИеыЗЂЩњЦЋзЊ |
II | NaHSO3ШмвК(pHЁж5) | БеКЯПЊЙиКѓСщУєЕчСїМЦжИеыЮДЗЂЩњЦЋзЊ |
(1)ХфжЦFeCl3ШмвКЪБЃЌЯШНЋFeCl3ШмгкХЈбЮЫсЃЌдйЯЁЪЭжСжИЖЈХЈЖШЁЃНсКЯЛЏбЇгУгяЫЕУїХЈбЮЫсЕФзїгУЃКЁЃ
(2)МзЭЌбЇЬНОПЪЕбщIЕФЕчМЋВњЮя______________ЁЃ
ЂйШЁЩйСПNa2SO3ШмвКЕчМЋИННќЕФЛьКЯвКЃЌМгШы______________ЃЌВњЩњАзЩЋГСЕэЃЌжЄУїВњЩњСЫ![]() ЁЃ
ЁЃ
ЂкИУЭЌбЇгжЩшМЦЪЕбщЬНОПСэвЛЕчМЋЕФВњЮяЃЌЦфЪЕбщЗНАИЮЊ______________ЁЃ
(3)ЪЕбщIжаИКМЋЕФЕчМЋЗДгІЪНЮЊ______________ЁЃ
ЃЈввЭЌбЇЕФЪЕбщЃЉ
ввЭЌбЇНјвЛВНЬНОПFeCl3ШмвКгыNaHSO3ШмвКФмЗёЗЂЩњЗДгІЃЌЩшМЦЁЂЭъГЩЪЕбщВЂМЧТМШчЯТЃК
зАжУ | БрКХ | ЗДгІЪБМф | ЪЕбщЯжЯѓ |
| III | 0~1 min | ВњЩњКьЩЋГСЕэЃЌгаДЬМЄадЦјЮЖЦјЬхвнГі |
1~30 min | ГСЕэбИЫйШмНтаЮГЩКьЩЋШмвКЃЌЫцКѓШмвКж№НЅБфЮЊГШЩЋЃЌжЎКѓМИКѕЮоЩЋ | ||
30 minКѓ | гыПеЦјНгДЅВПЗжЕФЩЯВуШмвКгжБфЮЊЧГКьЩЋЃЌЫцКѓж№НЅБфЮЊЧГГШЩЋ |
(4)ввЭЌбЇШЯЮЊДЬМЄадЦјЮЖЦјЬхЕФВњЩњдвђгаСНжжПЩФмЃЌгУРызгЗНГЬЪНБэЪОЂкЕФПЩФмдвђЁЃ
Ђй Fe3+ЃЋ3![]()
![]() Fe(OH)3 ЃЋ3SO2ЃЛЂк______________ЁЃ
Fe(OH)3 ЃЋ3SO2ЃЛЂк______________ЁЃ
(5)ВщдФзЪСЯЃКШмвКжаFe3+ЁЂ![]() ЁЂOHЃШ§жжЮЂСЃЛсаЮГЩКьЩЋХфКЯЮяВЂДцдкШчЯТзЊЛЏЃК
ЁЂOHЃШ§жжЮЂСЃЛсаЮГЩКьЩЋХфКЯЮяВЂДцдкШчЯТзЊЛЏЃК
![]()
ДгЗДгІЫйТЪКЭЛЏбЇЦНКтСНИіНЧЖШНтЪЭ1~30 minЕФЪЕбщЯжЯѓЃК______________ЁЃ
(6)НтЪЭ30 minКѓЩЯВуШмвКгжБфЮЊЧГКьЩЋЕФПЩФмдвђЃК______________ЁЃ
ЃЈЪЕбщЗДЫМЃЉ
(7)ЗжБ№ЖдБШIКЭIIЁЂIIКЭIIIЃЌFeCl3ФмЗёгыNa2SO3ЛђNaHSO3ЗЂЩњбѕЛЏЛЙдЗДгІКЭгаЙи(аДГіСНЬѕ)______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМюадаПУЬЕчГиЕФЙЄзїдРэЃКZn+2MnO2+2H2O![]() 2MnO(OH)+Zn(OH)2ЃЌЦфжаЕФЕчНтжЪШмвКЪЧKOHШмвКЁЃФГПЮЬтзщгУЗЯОЩЬњПЧЮоЙЏМюадаПУЬЕчГиЮЊдСЯЃЌжЦБИвЛжжаТаЭВФСЯЁЊЁЊMnxZn(1x)Fe2O4ЃЌЦфЙЄвеСїГЬШчЭМЫљЪОЃК
2MnO(OH)+Zn(OH)2ЃЌЦфжаЕФЕчНтжЪШмвКЪЧKOHШмвКЁЃФГПЮЬтзщгУЗЯОЩЬњПЧЮоЙЏМюадаПУЬЕчГиЮЊдСЯЃЌжЦБИвЛжжаТаЭВФСЯЁЊЁЊMnxZn(1x)Fe2O4ЃЌЦфЙЄвеСїГЬШчЭМЫљЪОЃК

(1)вбжЊMnxZn(1x)Fe2O4жаУЬдЊЫиЕФЛЏКЯМлгыЪЕбщЪвгУЖўбѕЛЏУЬжЦШЁТШЦјЪБЛЙдВњЮяжаЕФУЬЯрЭЌЃЌдђЬњдЊЫиЕФЛЏКЯМлЮЊ___________ЁЃ
(2)ЁАШмдќЁБЙЄађжаЯЁСђЫсгыЬњЗДгІЩњГЩЕФСђЫсбЧЬњПЩНЋ+3МлУЬЕФЛЏКЯЮяШЋВПЛЙдГЩMn2+ЃЌаДГіИУЗДгІЕФРызгЗНГЬЪНЃК_________________________________ЁЃ
(3)ЁАЕїЬњЁБЙЄађЕФФПЕФЪЧЕїећТЫвКжаЬњРызгЕФзмХЈЖШЃЌЪЙЦфжаН№ЪєдЊЫиЕФЮяжЪЕФСПжЎБШгыВњЦЗЕФЛЏбЇЪНMnxZn(1x)Fe2O4ЯрЗћКЯЁЃ
ЂйаДГіЁАЕїЬњЁБЙЄађжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЃК______________________ЁЂ_______ЁЃ
ЂкШєВтЕУТЫвКЕФГЩЗжЮЊc(Mn2+)+c(Zn2+)=a molЁЄL1ЃЌc(Fe2+)+c(Fe3+)=b molЁЄL1ЃЌТЫвКЬхЛ§ЮЊ1 m3ЃЌЁАЕїЬњЁБЙЄађжаЃЌашМгШыЕФЬњЗлжЪСПЮЊ___________kg(КіТдШмвКЬхЛ§БфЛЏЃЌгУКЌaЁЂbЕФДњЪ§ЪНБэЪО)ЁЃ
(4)дкЁАбѕЛЏЁБЙЄађжаЃЌМгШыЫЋбѕЫЎЕФФПЕФЪЧАб Fe 2+ бѕЛЏЮЊ Fe 3+ЃЛЩњВњЙ§ГЬжаЗЂЯжЪЕМЪЯћКФЫЋбѕЫЎЕФСПДѓгкРэТлжЕЃЌЦфПЩФмдвђГ§ЮТЖШЭтЃЌжївЊЪЧ______________________ЁЃ
(5)гУАБЫЎЁАЕїpHЁБКѓЃЌОЁАНсОЇЁБЁЂЁАЙ§ТЫЁБПЩЕУЕНВњЦЗКЭТЫвКCЃЌДгТЫвКCжаЛЙПЩЗжРыГівЛжжЕЊЗЪЃЌИУЕЊЗЪЕФШмвКжаРызгХЈЖШгЩДѓЕНаЁЕФХХађЮЊ_______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃЮТЯТЃЌЯђФГХЈЖШЕФH2C2O4ШмвКжаж№ЕЮМгШывбжЊХЈЖШЕФNaOHШмвКЃЌШєpCБэЪОШмвКжаШмжЪЮЂСЃЕФЮяжЪЕФСПХЈЖШЕФИКЖдЪ§ЃЌдђЫљЕУШмвКжаpC(H2C2O4)ЃЌpC(HC2O4Ѓ)ЁЂpC(C2O42Ѓ)гыШмвКpHЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃвбжЊЃКH2C2O4![]() HC2O4Ѓ+H+ Ka1ЃЛHC2O4Ѓ
HC2O4Ѓ+H+ Ka1ЃЛHC2O4Ѓ![]() C2O42Ѓ+H+ Ka2ЁЃдђЯТСаЫЕЗЈе§ШЗЕФЪЧ
C2O42Ѓ+H+ Ka2ЁЃдђЯТСаЫЕЗЈе§ШЗЕФЪЧ

A. ЕБpH=3ЪБЃЌШмвКжаc(HC2O4Ѓ)<c(C2O42Ѓ)=c(H2C2O4)
B. pHгЩ3діДѓЕН5.3ЕФЙ§ГЬжаЃЌЫЎЕФЕчРыГЬЖШж№НЅМѕаЁ
C. ГЃЮТЯТЃЌKa2=10Ѓ5.3
D. ГЃЯТЫцзХpHЕФдіДѓЃКc2(HC2O4Ѓ)/[c(H2C2O4)c(C2O42Ѓ)] ЕФжЕЯШдіДѓКѓМѕаЁ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭММзЪЧРћгУвЛжжЮЂЩњЮяНЋЗЯЫЎжаЕФФђЫи[CO(NH2)2]ЕФЛЏбЇФмжБНгзЊЛЏЮЊЕчФмЃЌВЂЩњГЩЛЗОГгбКУЮяжЪЕФзАжУЃЌЭЌЪБРћгУДЫзАжУЕФЕчФмдкЬњЩЯЖЦЭЁЃЯТСаЫЕЗЈжае§ШЗЕФЪЧ
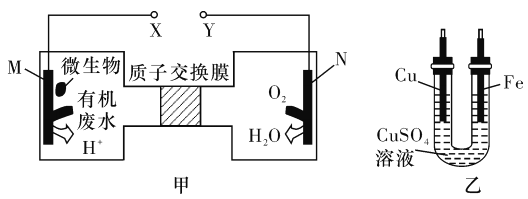
A. ЭЕчМЋгІгыXЯрСЌНг
B. H+ОЙ§жЪзгНЛЛЛФЄгЩгвЯђзѓвЦЖЏ
C. ЕБNЕчМЋЯћКФ0. 25 molЦјЬхЪБЃЌдђЬњЕчМЋдіжи16 g
D. MЕчМЋЗДгІЪНЃКCO(NH2)2+H2O-6e- =CO2Ёќ+N2Ёќ+6H+
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬьШЛЫЎДѓЖрКЌCa2+ЁЂMg2+ЁЂHCO3-ЕШРызгЃЌМгШШЛсВњЩњЫЎЙИЃЌЫЎЙИжавЛЖЈКЌгаCaCO3КЭMg(OH)2ЃЌПЩФмКЌгаMgCO3ЁЃ
(1)ЬьШЛЫЎжаЕФHCO3-РДздгкПеЦјжаЕФCO2ЁЃгУЯрЙиЗНГЬЪНБэЪОCO2ШмгкЫЎаЮГЩHCO3-ЕФЙ§ГЬЁЃ______________________________________________________________
(2)ЬьШЛЫЎжѓЗаЪБЃЌЦфжаЮЂШмЕФMgCO3зЊЛЛГЩФбШмЕФMg(OH)2ЃЌаДГіЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЁЃ____________________________________________
ЮЊШЗЖЈФГЫЎЙИбљЦЗЕФГЩЗжЃЌРћгУCaCO3ЁЂMgCO3ЁЂMg(OH)2ИпЮТЗжНтЕФаджЪЃЌОЋШЗГЦСП5.000gЫЎЙИбљЦЗЃЌгУШчЯТЭМзАжУНјааЪЕбщЁЃ
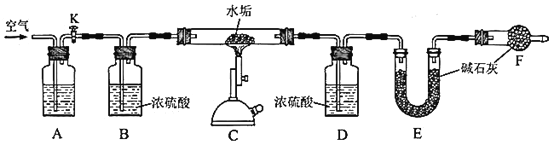
(3)AжаЪЂЗХЕФЪдМСЪЧ__________ЁЃзАжУFЕФзїгУЪЧ_________________________ЁЃЗДгІНсЪјКѓЃЌашвЊдйЭЈШывЛЖЮЪБМфЕФПеЦјЃЌФПЕФЪЧ_______________________ЁЃ
(4)РћгУЩЯЪізАжУВтЖЈЫЎЙИжаMg(OH)2ЕФКЌСПЪБЃЌашвЊВтСПЕФЪ§Онга__________ЁЃ
(5)ЪЕбщВтЕУзАжУEдіжи2.200gЃЌЧыЮЪЫЎЙИбљЦЗжаЪЧЗёКЌгаMgCO3?ХаЖЯвРОнЪЧ_______________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭЈЙ§КЃЫЎСРЩЙПЩЕУДжбЮ,ДжбЮжаГ§КЌгаNaClЭт,ЛЙКЌга![]() вдМАФрЩГЕШдгжЪЁЃжЦБИОЋбЮЕФИїВНВйзїСїГЬШчЯТ:
вдМАФрЩГЕШдгжЪЁЃжЦБИОЋбЮЕФИїВНВйзїСїГЬШчЯТ:

(1)дкЕкЂпВНеєЗЂЙ§ГЬжавЊгУВЃСЇАєНСАш,ФПЕФЪЧ__________ЁЃ
(2)ЕкЂкЁЂЂмВНВйзїЕФФПЕФЗжБ№ЪЧГ§ШЅДжбЮжаЕФ![]() КЭ
КЭ![]() ,ашвЊМгШыЕФЪдМСвРДЮЪЧ________(ЬюЛЏбЇЪН)ЁЃ
,ашвЊМгШыЕФЪдМСвРДЮЪЧ________(ЬюЛЏбЇЪН)ЁЃ
(3)ЕкЂоВНВйзїжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________ЁЃ
(4)дкЕкЂлВНВйзїжа,бЁдёЕФГ§дгЪдМСВЛФмЪЧKOHШмвК,РэгЩЪЧ_____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПКЃЫЎЪЧОоДѓЕФзЪдДБІПтЃЌКЃЫЎЕЛЏМАЦфзлКЯРћгУОпгаживЊвтвхЁЃ

ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉВНжшIжаЃЌДжбЮжаКЌгаCa2+ЁЂMg2+ЁЂSO42-ЕШдгжЪРызгЃЌДжбЮОЋжЦЙ§ГЬжавЊЪЙгУNa2CO3ШмвКЃЌЧыаДГіМгШыNa2CO3ШмвККѓЯрЙиЛЏбЇЗДгІЕФРызгЗНГЬЪНЃК_________________________ЁЃ
ЃЈ2ЃЉКЃЫЎЬсфхЃЌжЦЕУ1molBr2жСЩйашвЊЯћКФ_________molCl2ЁЃВНжшЂѓШєгУNa2SO3ЫЎШмвКЮќЪеBr2ЃЌгаЙиЗДгІЕФРызгЗНГЬЪНЮЊ_________ЁЃ
ЃЈ3ЃЉгУЫФТШЛЏЬМПЩвдНЋЩњГЩЕФфхЬсШЁГіРДЃЌРћгУСЫфхЕФ________аджЪЃЌЮЊСЫГ§ШЅВњЮяжаВаСєЕФЩйСПCl2ЃЌПЩЯђЦфжаМгШы_________ШмвКЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊЃКp[c(HX)/c(X-)]=-lg[c(HX)/c(X-)]ЁЃЪвЮТЯТЃЌЯђ0. 10 mol/LHXШмвКжаЕЮМг0.10 mol/L NaOHШмвКЃЌШмвКpHЫцp[c(HX)/c(X-)]БфЛЏЙиЯЕШчЭМЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
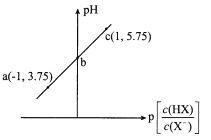
A. ШмвКжаЫЎЕФЕчРыГЬЖШЃКa<b<cB. ЭМжаbЕузјБъЮЊ(0ЃЌ4.75)
C. cЕуШмвКжаЃКc(Na+) =l0c(HX)D. ЪвЮТЯТHXЕФЕчРыГЃЪ§ЮЊ10-4. 75
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com