
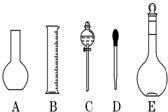
 ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺���� ��1����������һ�����ʵ���Ũ����Һ�õ�����ѡ����������Һ�����ѡ������ƿ�Ĺ��
��2������ƿ����ϡ��Ũ��Һ�����������ܽ���塢������Һ�ȣ�
��3������������Һ���ѡ���������ƿ������m=CVM������Ҫ�������Ƶ����������ݹ�ʽc=n/V�����Ը���Ӱ��n��V��������������������
��4��������Һϡ��ǰ���������ʵ����ʵ������䣬��������18mol/L��Ũ�����������ݴ�ѡ����Ͳ���
��� �⣺��1������һ�����ʵ���Ũ����Һ�õ������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����Ҫ����������ƿ�ͷ�Һ©��������C�������Ƿ�Һ©����Ҫ����450mL��ҺӦѡ��500mL����ƿ��
�ʴ�Ϊ��AC����Һ©����500��
��2������ƿֻ����������һ�����ȷŨ�ȵ���Һ���������ƻ��������ƿ������µ����������Һ�壬����ϡ�ͻ��ܽ�ҩƷ���������������ܽ�������ʣ�
��ѡ��BCD��
��3������ʱ���ӿ̶��ߣ�������Һ���Vƫ����ҺŨ��ƫС����ҺŨ��С��0.1mol/L����NaOH��Һδ��ȴ��ת��������ƿ���ݣ���ָ����º���Һ���ƫС������������ҺŨ�ȴ���0.1mol/L��
�ʴ�Ϊ��С�ڣ����ڣ�
��4��������Һϡ��ǰ���������ʵ����ʵ������䣬����18mol/L��Ũ��������ΪVL����18mol/L��VL=0.5mol/L��0.5L��V=0.0139L��13.9mL��ѡ��25mL��Ͳ��
�ʴ�Ϊ��13.9��25��
���� ������Ҫ����һ�����ʵ���Ũ����Һ��������ϡ�ͣ�ע������ƿ����ѡ���ʹ��ע������Ϊ�״��㣬�ѶȲ���


 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
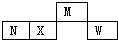 ����������Ԫ��M��N��X��W��Ԫ�����ڱ��е����λ����ͼ��ʾ��NԪ�صĵ��ʳ������뵼����ϣ������ж���ȷ���ǣ�������
����������Ԫ��M��N��X��W��Ԫ�����ڱ��е����λ����ͼ��ʾ��NԪ�صĵ��ʳ������뵼����ϣ������ж���ȷ���ǣ�������| A�� | ԭ�Ӱ뾶�Ĵ�С��W��X��M | |
| B�� | ��̬�⻯����ȶ��ԣ�N��X��M | |
| C�� | ��Ӧ�ĺ����������ǿ����W��X��N | |
| D�� | W�ֱ���N��X�γɵĻ������еĻ�ѧ����Ϊ���Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
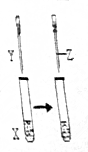 ����ͼ��ʾװ�ý���ʵ�飬ʵ��������ȷ���ǣ�������
����ͼ��ʾװ�ý���ʵ�飬ʵ��������ȷ���ǣ������� | X | Y | Z | ʵ������ | |
| A | ���� | NaOH | ���� | ����Һ����壬���ֱ���� |
| B | FeCl3 | KSCN | KCl���� | ��Һ��Ϊ��ɫ������ɫ�ӻ� |
| C | KI | ������ˮ | �Ҵ� | ��Һ��Ϊ��ɫ������Һ�ֲ㣬�ϲ�Ϊ�Ϻ�ɫ���²�Ϊ��ɫ |
| D | Na2SO3 | Ba��NO3��2 | ���� | ���ɰ�ɫ������������ܽ⣬�д����������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4�� | B�� | 6�� | C�� | 8�� | D�� | 10�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
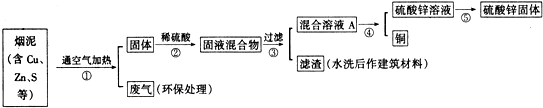
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | Cu | Zn | S |
| 1 | 10.3% | 5.0% | 1.2% |
| 2 | 11.5% | 4.9% | 1.8% |
| 3 | 12.4% | 10.3% | 0.9% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/LCH3COOH��Һ����ˮϡ�����У���������Ũ�Ⱦ���С | |
| B�� | Ũ�Ⱦ�Ϊ0.1mol/L��NaF��CH3COONa��Һ��Ƚϣ�CH3COONa��ҺpH�� | |
| C�� | ��ӦHF+CH3COONa�TNaF+CH3COOH���Է��� | |
| D�� | NaF��Һ�м�����NaOH���壬��Һ��c��F-����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
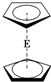 E��C5H5��2�Ľṹ��ͼ��������ԭ�ӵĻ�ѧ������ȫ��ͬ������������ȴ�������Ϊ���ĽṹΪ
E��C5H5��2�Ľṹ��ͼ��������ԭ�ӵĻ�ѧ������ȫ��ͬ������������ȴ�������Ϊ���ĽṹΪ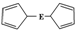 ��1H�˴Ź����ܹ����������ֽṹ����1H�˴Ź������У�����Ľṹ����ȷ�Ľṹ1H�˴Ź����ķ�ֱ�Ϊ��������
��1H�˴Ź����ܹ����������ֽṹ����1H�˴Ź������У�����Ľṹ����ȷ�Ľṹ1H�˴Ź����ķ�ֱ�Ϊ��������| A�� | 5��5 | B�� | 3��5 | C�� | 5��1 | D�� | 3��1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com