
��3�֣���úת��Ϊˮú����CO��H2�Ļ�����壩��ͨ����ѧ������úת��Ϊ�ྻȼ�ϵķ���֮һ��úת��Ϊˮú������Ҫ��ѧ��ӦΪ��C(s) + H2O(g)��CO(g) + H2(g)����H1 �� ��֪��
��2H2(g) + O2(g) �� 2H2O(g)����H2����483.6kJ��mol��1
��2C(s) + O2(g) �� 2 CO(g)�� ��H3����221.0kJ��mol��1
��������Ȼ�ѧ����ʽ������ó���H1 �� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
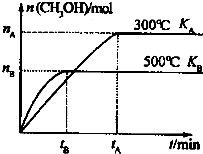 CO��H2�����ǵ�����������ȷ���������أ�
CO��H2�����ǵ�����������ȷ���������أ�| 1 |
| 2 |
| 1 |
| 2 |
| 2nB |
| 3tB |
| 2nB |
| 3tB |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
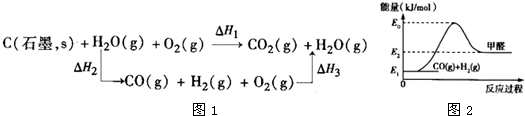
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������?�ɵ���ˮ������������ˮ������ | B������ֱ����ʹ�öԴ������������ƻ����õķ����� | C��?�á���ɫ��ѧ�����գ�ʹԭ�Ͼ�����ת��Ϊ����Ҫ������ | D����úת��Ϊˮú����ȼ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com