
��1��1.00 mol FeS2����ȫ��������Ҫ�������������״����Ϊ_________L��
��2��55 L����������FeS2��ȫ��Ӧ�����������ͬ��ͬѹ����Ϊ__________L��
��3���ÿ�������FeS2���������������У�O2�ĺ���Ϊ0.0800,����SO2�ĺ�����
(4)������FeS2��������������Ϊ100 L������O2Ϊa L��SO2Ϊb L����д��a��b�Ĺ�ϵʽ��������ͼ�л���a��b�Ĺ�ϵ���ߣ�����FeS2ʱ����������20%����

˵����Ϊ������ͼ����������13b��ʾ��
��1��308 (2)52 (3)0.0923
(4)��13b=200-10a ����ͼ

��������1�����ݷ�Ӧ�Ļ�ѧ����ʽ��֪��1.00 mol FeS2����ȫ������Ҫ����11/4 mol��ת��Ϊ�������������״����Ϊ��11/4 mol��22.4 L��mol-1��0.200=308 L��
��2�����ò��������跴Ӧ���ٵ����Ϊx��
4FeS2+11O2====8SO2+2Fe2O3 ��V
11 3
55 L��0.200 x
��ã�x=3 L���������������Ϊ��55 L-3 L=52 L��
��3����SO2Ϊy(���������������4��(y/8��11+0.0800)=1-0.0800-y,��ã�y=0.0923��
��4����100 L������������N2Ϊ��100-a-b) L,������b L SO2��O2Ϊ11b/8 L,���У���100-a-b)��(11b/8+a)=4��1,��ã�13b=200-10a��
�ڸ���������ϵʽ����ͼ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
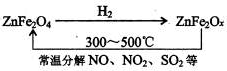
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
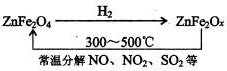
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���Ĵ�ʡ������ѧ�߿���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com