
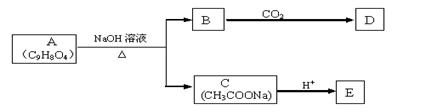
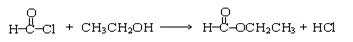
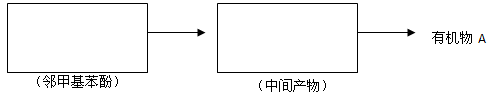
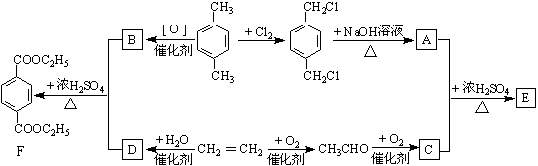
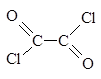 ���������л���D��Ӧ�Ļ�ѧ����ʽ________________________________________________________________________��
���������л���D��Ӧ�Ļ�ѧ����ʽ________________________________________________________________________��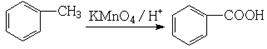
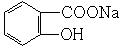
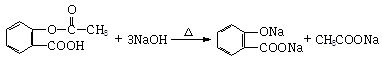 + 2H2O
+ 2H2O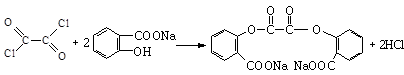 ��
��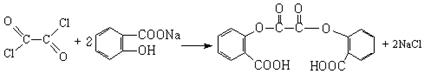
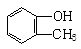
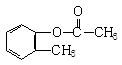
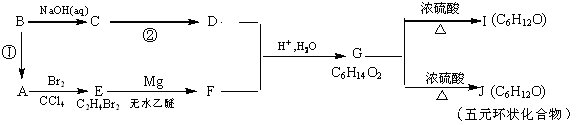
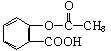 ����B��
����B��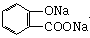 �������Ȼ�������ǿ��̼��ģ���̼�������ǿ�ڷ��ǻ��ģ�����D�Ľṹ��ʽ��
�������Ȼ�������ǿ��̼��ģ���̼�������ǿ�ڷ��ǻ��ģ�����D�Ľṹ��ʽ�� ��
��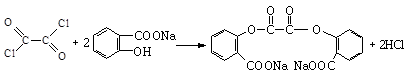 ��
��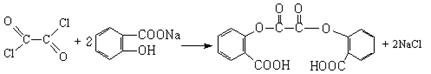 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
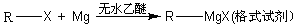
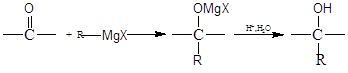
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 D��
D�� | A��ͬһ������ | B��ͬһ������ | C��ͬϵ�� | D��ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
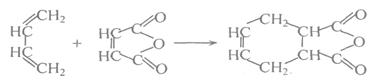
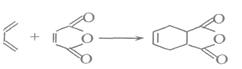
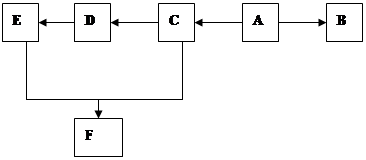
 X��CH2һCH=CH��CH2һX(X2=F2��C12��Br2)��
X��CH2һCH=CH��CH2һX(X2=F2��C12��Br2)��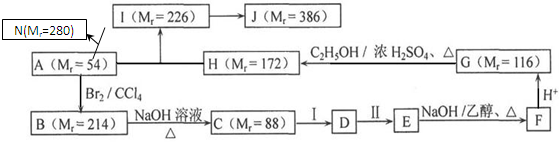
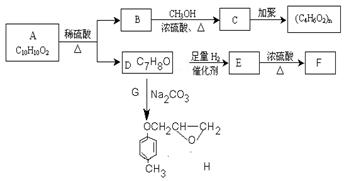
 C�ķ�Ӧ���� ��
C�ķ�Ӧ���� �� H�� ��
H�� �� N�� ��
N�� ��| A��ȡ����Ӧ | B���ӳɷ�Ӧ | C���Ӿ۷�Ӧ | D����ȥ��Ӧ E���кͷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
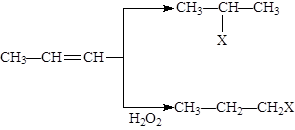 ��XΪ±��ԭ�ӣ�
��XΪ±��ԭ�ӣ�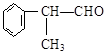 �����ʣ���������һ�����ϡ�
�����ʣ���������һ�����ϡ�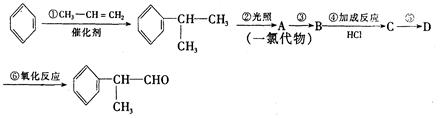
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
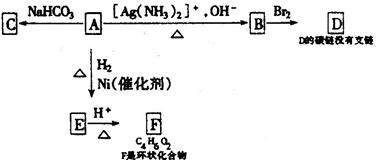
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com