
 �����ơ����˽𡱣���ѧ�ɷ���FeS2�������������徧ϵ������������Ϊ�ǻ������ʣ��¶����ߺ��û��ã��ڿ����������������������Ͷ���������Ҫ���ڽӴ����������
�����ơ����˽𡱣���ѧ�ɷ���FeS2�������������徧ϵ������������Ϊ�ǻ������ʣ��¶����ߺ��û��ã��ڿ����������������������Ͷ���������Ҫ���ڽӴ�������������� ��1����FeS2�е�S2-��2��Sԭ�ӵõ�1�������γɵ������ӣ�
��2��Fe3+��Fe2+�ȶ���3d���Ӳ�ͬ������Ϊ�ȶ��ṹ��
��3��H2S2������Hԭ�Ӻ�Sԭ��֮���Լ��Թ��ۼ���ϣ�Sԭ�Ӻ�Sԭ��֮���ԷǼ��Թ��ۼ���ϣ�
SO2������Sԭ�ӵļ۲���Ӷ���=2+$\frac{6-2��2}{2}$=3���ӻ������ĿΪ3��
��Ϊ�ȵ�����������ǵĿռ乹����ͬ����ԭ��������ȡ��۵���������ͬ������Ϊ�ȵ����壻
��4������H2SO4��S�Ļ��ϼ�Ϊ+6�ۣ�H2SO3��SΪ+4�ۣ�����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
��5�����ݾ�̯�����㣬�ٸ����ܶ�$��=\frac{m}{V}$���㣮
��� �⣺��1����FeS2�е�S2-��2��Sԭ�ӵõ�1�������γɵ������ӣ��ʵ����Ų�ʽΪ[Ar]3d6��1s22s22p63s23p63d6���ʴ�Ϊ��[Ar]3d6��1s22s22p63s23p63d6��
��2����ΪFe3+�Ļ�̬�����Ų�ʽΪ[Ar]3d5����������Ӿ������ȶ�״̬��Fe2+�Ļ�̬�����Ų�ʽΪ[Ar]3d6��3d������Ӳ����ȶ�״̬������Fe3+��Fe2+Ҫ�����ȶ�
���ʴ�Ϊ����ΪFe3+�Ļ�̬�����Ų�ʽΪ[Ar]3d5����������Ӿ������ȶ�״̬��Fe2+�Ļ�̬�����Ų�ʽΪ[Ar]3d6��3d������Ӳ����ȶ�״̬������Fe3+��Fe2+Ҫ�����ȶ���
��3��H2S2������Hԭ�Ӻ�Sԭ��֮���Լ��Թ��ۼ���ϣ�Sԭ�Ӻ�Sԭ��֮���ԷǼ��Թ��ۼ���ϣ����ڦҼ���SO2������ԭ��֮���γ�2�Թ��õ��Ӷԣ����ЦҼ����м���Sԭ�Ӽ۲���Ӷ���=2+$\frac{6-2��2}{2}$=3����Sԭ���ӻ������ĿΪ3��Sԭ�Ӳ�ȡsp2�ӻ���
��Ϊ�ȵ�����������ǵĿռ乹����ͬ����SO2������ͬ�ռ乹�͵���ΪH2O��H2S�ȣ�
�ʴ�Ϊ���Ҽ���sp2��H2O��H2S�ȣ�
��4������H2SO4��S�Ļ��ϼ�Ϊ+6�ۣ�H2SO3��SΪ+4�ۣ�����H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
�ʴ�Ϊ������H2SO4��S�������Ը���H2SO3��S�������ԣ������ǻ���Oԭ�ӵĵ���ƫ��S����ˮ���ӵ������¾��������H+�������Ը�ǿ��
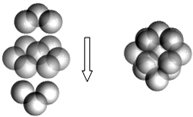
��5����ÿ��Fe2+��Χ����ĵȾ����S22-���Ӵ��������ģ�����6�����ʴ�Ϊ��6��
��һ�������ں���Fe2+��ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������S2-��ĿΪ12��$\frac{1}{4}$+1=4��һ������������m=$\frac{120g/mol��4}{{N}_{A}}$��һ���������V=a03�����ܶ�$��=\frac{m}{V}$=$\frac{120g/mol��4}{��0.54��1{0}^{-7}cm��^{3}��6.02��1{0}^{23}}$=5.06g•cm-3���ʴ�Ϊ��5.06��
���� ���⿼�������ʽṹ�����ʣ��漰��ѧ��������ļ��㡢��������Ų�ʽ��Ӧ�õ�֪ʶ�㣬��Щ֪ʶ�㶼�Ǹ߿��ȵ㣬ѧ����ݾ�̯��ȷ�����ʵĻ�ѧʽ���Ѷ��еȣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
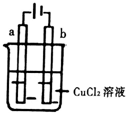
| A�� | �缫a����ʺ�ɫ | |
| B�� | �缫b������ | |
| C�� | �����ɵ�Դ�������ص�������缫a | |
| D�� | ����·��ͨ��0.2mol����ʱ��������b�缫��������6.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ӻ������ж��������Ӽ� | |
| B�� | ���ӻ������е�������ֻ���ǽ������� | |
| C�� | ���ۻ�����Ҳ�ܺ������Ӽ� | |
| D�� | ���ӻ����ﲻ�ܺ��й��ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
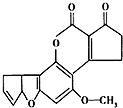 ����ù��AFTB1����ͼ������Ⱦ��ʳ�����ù�أ��������������ڻ���ù�ص������»ᷢ��ͻ�䣬��ת��ɸΰ��Ŀ����ԣ���1mol����ù����Ӧ��H2��NaOH��������ֱ��ǣ�������
����ù��AFTB1����ͼ������Ⱦ��ʳ�����ù�أ��������������ڻ���ù�ص������»ᷢ��ͻ�䣬��ת��ɸΰ��Ŀ����ԣ���1mol����ù����Ӧ��H2��NaOH��������ֱ��ǣ�������| A�� | 6mol��2mol | B�� | 7mol��2mol | C�� | 6mol��1mol | D�� | 7mol��1mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���տ�����0.2 mol NH3 | |
| B�� | ����ѹǿ��ʹ��Ӧ���ʼ�С | |
| C�� | ���ͷ�Ӧ��ϵ���¶��ܼӿ췴Ӧ���� | |
| D�� | ���������ټ���N2���ܼӿ췴Ӧ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ũ���ᡢŨ����Ļ������60��ˮԡ���������·�Ӧ������������ | |
| B�� | �����Ը�����ؿ��Լ���CH2=CH-CHO��̼̼˫�� | |
| C�� | ��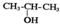 ��NaOH�Ĵ���Һ�����Ʊ�CH3-CH=CH2 ��NaOH�Ĵ���Һ�����Ʊ�CH3-CH=CH2 | |
| D�� | ��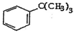 ��ͨ�����������Ը��������Һ���Եõ� ��ͨ�����������Ը��������Һ���Եõ�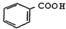 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��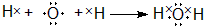 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com