
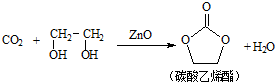
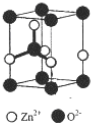
���� ��1��Znԭ�Ӻ��������Ϊ30�������������ԭ����д��������Ų�ʽ�����þ�̯�����㾧����O2-����Ŀ��
��2�������������������ṩ�չ���������ṩ�¶Ե��ӣ�
��3��̼����ϩ����������Cԭ���γ�3���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ3���Ǽ���Cԭ���γ�4���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ4��
1mol̼����ϩ���к���10mol���ۼ���
��4��ԭ��������ȡ��۵���������ȵ�����Ϊ�ĵȵ����壮
��� �⣺��1��Znԭ�Ӻ��������Ϊ30����������Ų�ʽΪ1s22s22p63s23p63d104s2��ÿ��ZnO�����к�O2-����ĿΪ1+8��$\frac{1}{8}$=2��
�ʴ�Ϊ��1s22s22p63s23p63d104s2��2��
��2��Zn2+���пչ����H2O���йµ��Ӷԣ���Zn2+�ṩ�չ����H2O�ṩ�µ��Ӷԣ��γ�������[Zn��H2O��6]2+��
�ʴ�Ϊ��Zn2+��
��3��̼����ϩ����������Cԭ���γ�3���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ3��̼ԭ�Ӳ�ȡsp2�ӻ����Ǽ���Cԭ���γ�4���Ҽ���û�й¶Ե��ӣ��ӻ������ĿΪ4��̼ԭ�Ӳ�ȡsp3�ӻ���
1mol̼����ϩ���к���10mol���ۼ������еļ���ĿΪ��10��6.02��1023��
�ʴ�Ϊ��sp2��sp3 ��10��6.02��1023��
��4��������X-��CO2��Ϊ�ȵ�������X-�ں����ۼ����������X-�Ļ�ѧʽΪ HF2-��
�ʴ�Ϊ��HF2-��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����������㡢�����ӻ���ʽ����ѧ�����ȵ�����ȣ�ע��Ի���֪ʶ���������գ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
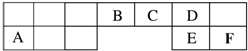
| B | C |
| D |
| A�� | �����ӵİ뾶��С��ϵ��C��E | |
| B�� | DԪ�ص���̬�⻯���CԪ�ص���̬�⻯���ȶ� | |
| C�� | ��A��B��C����Ԫ����ɵ����ӻ������У����������Ӹ�����Ϊ1��1 | |
| D�� | ��C��D��E����Ԫ����ɵĻ������Һ�����Ի���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
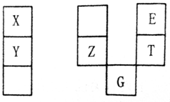 X��Y��Z��E��T��GԪ�������ڱ��е����λ���±���T������������ˮ������ǿ��ˮ�ԣ�Y��Z��ͬһ���ڣ�Yԭ������������ڲ������ͬ�������������ж���ȷ���ǣ�������
X��Y��Z��E��T��GԪ�������ڱ��е����λ���±���T������������ˮ������ǿ��ˮ�ԣ�Y��Z��ͬһ���ڣ�Yԭ������������ڲ������ͬ�������������ж���ȷ���ǣ�������| A�� | E��G��ԭ���������25 | |
| B�� | �����£�X��Y�ĵ��ʾ�����ˮ���ҷ�Ӧ | |
| C�� | TE2���۵����ZE2���� | |
| D�� | E��T��ZԪ�ص�ԭ�Ӱ뾶�����ǵ���̬�⻯������ȶ��Ծ����ε��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ˮ�еμ�NaHCO3�������ݲ�����˵����ˮ�к���HCl��HClO | |
| B�� | ��FeCl2��Һ�еμ���ˮ����Һ����ػ�ɫ��˵����ˮ�к���HClO | |
| C�� | ��ˮ��dz����ɫ��˵����ˮ�к���Cl2 | |
| D�� | ����ˮ�еμ������ữ��AgNO3��Һ��������ɫ������˵����ˮ�к�Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ca��ClO��2��Һ�У�c��Ca2+����c��ClO-����c��OH-����c��H+�� | |
| B�� | ������������ʵ���Ũ�ȵ�NaX������HX��ϣ�c��Na+ ��=c��X-����c��OH-��=c��H+�� | |
| C�� | �����£���25mL0.2mol/L��������100mL0.1mol/L�İ�ˮ��ϣ�������Һ�У�c��Cl-����c��NH4+����c��NH3•H2O����c��OH-����c��H+�� | |
| D�� | ��0.1mol/L��Na2S��Һ��0.1mol/L��NaHS��Һ�������ϣ�������Һ�У�c��S2-��+2c��OH-��=2c��H+��+c��HS-��+3c��H2S�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com