
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��1����Ũ��������ʵ���Ũ��Ϊ__________mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ�� ���Ķ��ٶ��仯����__________��
A����Һ��H2SO4�����ʵ��� B����Һ��Ũ��
C����Һ��SO42������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����480 mL���ʵ���Ũ��Ϊ0.2 mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ________mL����Ũ����������ơ�
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��һ�����������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У�����ʵ�����ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���_________
A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B��ϡ���õ��ձ��Ͳ�����δϴ��
C��ϴ��������ƿδ�����������������Һ
D����Һע������ƿǰû�лָ������¾ͽ��ж���
E������ʱ���ӹ۲찼Һ��
F����ˮ�����̶��ߺ��ý�ͷ�ι����������Һ��
���ֽ�100mL��������300mL 0.4mol/LCuSO4��Һ��ϣ�����仯���Բ��ƣ�������Һ��SO42�������ʵ���Ũ����_________mol/L��
19����1��18.4 ��2��BD��3����5.4 ��BCAFED ��ADE ��0.35
���������������1����c="1000�Ѧ�/M" =18.4mol/L��
��2�� ��Һ��Ũ�ȡ���Һ���ܶȲ�����ȡ��Һ��������ı䡣
��3��������Ҫ��ȡ��Һ�������Vml:����480 mL��0.2 mol/L="V��18.4" mol/L��ã�V ="5.4" mL ������˳��Ϊ�����㡢�������ܽ⡢ϴ�ӡ�ת�ơ����ݡ�ҡ�ȡ��ݴ˿�ѡ��𰸡�
��A������ȡŨ����������������ֵ��B������ʧ���ʣ�C������Ӱ�죻D����Һ��������������Һ���С������ֵ��E������ˮ�����С������ֵ��F������ʧ���ʡ�����ʹ��Һ���ʵ���Ũ��ƫ�ߵ���ADE��
��n(SO42��)="0.1L��0.2" mol/L+0.3L��0.4 mol/L="0.12" mol.����C="n/V=0.12mol/0.4L=0.35" mol/L.
���㣺һ�����ʵ���Ũ�ȵ���Һ�����úͼ��㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ϳɰ���ҵ���������õĦ�Fe��������Ҫ�ɷ���FeO��Fe2O3��
��1��ijFeO��Fe2O3������У������������ʵ���֮��Ϊ4��5������Fe2����Fe3�����ʵ���֮��Ϊ________��
��2����������Fe2����Fe3�������ʵ���֮��Ϊ1��2ʱ�����������ߣ���ʱ����������������������������Ϊ________����С����ʾ������2λС������
��3����Fe2O3Ϊԭ���Ʊ������������������м�������̼�ۣ��������·�Ӧ��2Fe2O3��C 4FeO��CO2����
4FeO��CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ٱ�״���£�1���ˮ��������ܽ�500�����HCl��
�ڱ���NaCl��Һ��Ũ��ԼΪ5.00 mol��L��1��
�ڱ�״���£���448LHCl��������1 Lˮ�У�������ҺA���ܶ�Ϊ1.20 g��cm-3������ҺA��HCl�����ʵ���Ũ��Ϊ ��(�����������ȡ��λ��Ч����)
��1����ʹCl��Ũ������ҺA�е�Cl��Ũ����ȣ�����1 L NaCl������Һ�л�Ӧ�ܽ�Լ L��״����HCl���� (��Һ����仯���Բ���)��
��2��ȡ10.0mL��ҺAϡ�ͳ�500mL��ҺB������ҺB��HCl�����ʵ���Ũ��Ϊ ��
��3������ҺB�����ƹ����У�ʹ��ǰ�������Ƿ�©Һ�������� ���������Ʋ����������ҺBŨ��ƫ�͵���_______________(ѡ�����)��
a.����ƿ������ˮϴ�Ӻ�δ����
b.��ȡ��ҺA����Ͳ������ˮϴ�Ӻ�δ����
c.����ʱ������Һ���ˮ���̶���
d.��ˮ����ʱҺ�治�������̶��ߣ������ý�ͷ�ι���������ˮʹҺ��պô�̶���
e.�ձ�����Һ��������ƿ��δ��ˮϴ���ձ��Ͳ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ����ˮ�ʼ��վ����1000mL 1 mol��L��1NaOH��Һ�Ա�ʹ�á�
��1����ͬѧӦѡ��___________mL������ƿ��
��2���������������ͼ��ʾ������ͼ����Ӧ����ͼ�е� (��ѡ����ĸ)֮�䡣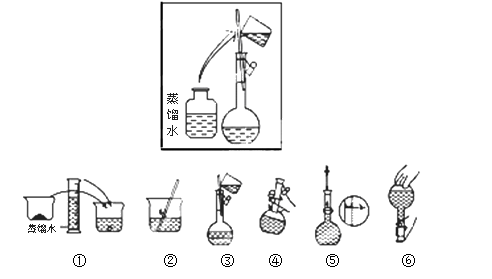
A������ۡ����� B������ڡ��� ���� C�������
��3����ͬѧӦ��������ƽ��ȡNaOH���� g��������Ϊ33.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ�� ����ѡ����ĸ����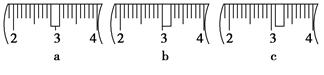
��4�����в�����������Һ��Ũ�ȴ�С�к�Ӱ�� (�ƫ����ƫС������Ӱ�족)��
�ٶ���ʱ�����Ӷ�����Ũ�Ȼ� ��
��ת����Һ�����У�����Һ�彦������Ũ�Ȼ� ��
������ƿδ���Ũ�Ȼ� ��
�ܶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ�Ũ�Ȼ� ��
��5��������Һ��ʵ�ʲ��������У�����Ҫ�죬�������� ����ʹ���Ƶ�NaOH��Һ��Ũ�ȱ�1 mol��L��1 �����С������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����m gij���壬������ԭ�ӷ��ӹ��ɣ�����Ħ������ΪM g��mol��1����
��1������������ʵ���Ϊ____ ____��
��2����������������ԭ������Ϊ________����
��3���������ڱ�״���µ����Ϊ________L��
��4������������1 Lˮ��(�����Ƿ�Ӧ)������Һ�����ʵ���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڻ�ƿ�м��롰�ʻ����ʼ��������ӳ��ʻ����������±���500mL���ʻ����ʼ����к��еijɷ֣��Ķ���ش��������⣺
| �ɷ� | ������g�� | Ħ��������g��mol-1�� |
| ���� | 25.00 | 342 |
| ����� | 0.87 | 174 |
| ��˾ƥ�� | 0.17 | 180 |
| ������� | 0.316 | 158 |
| ������ | 0.075 | 170 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��8 g CH4�����ʵ���Ϊ ���ڱ�״������ռ�����ԼΪ ��
��2��100mL 1 mol��L-1Al2(SO4)3��Һ�к�Al3+���� mol����SO42-���� ����
��3��0.6mol O2��0.4mol O3����֮��Ϊ �����Ӹ���֮��Ϊ ��ԭ�Ӹ���֮�� ��������ͬ��ͬѹ�µ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й�����
�ش��������⣺
��1����Ũ������HCl�����ʵ���Ũ��Ϊ__________mol��L��1��
��2��������ƿ��ʹ�÷����У����в�������ȷ����____________��������ѡ��
| A��ʹ������ƿǰ�����Ƿ�©ˮ |
| B������ƿ��ˮϴ�������ô�����Һϴ�� |
| C��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
| D���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ� |
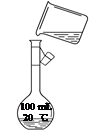
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ���ҳ����������������Ϊ36.5%���ܶ�Ϊ1.20g/�M3��
�Ŵ�Ũ��������ʵ���Ũ���Ƕ��٣�(��ʽ����)
������100mL3.00mol/L�����ᣬ������Ũ�������mL��(��ʽ����)
����Ũ�������Ƹ�ϡ������Ҫ������Щ���裨������˳����д��ţ��� ��
�ټ��� ��װƿ����50 mL��Ͳ��ȡһ�������Ũ�����ϴ�Ӣ���Һ��ϡ�͢߶��ݢ�ҡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com