
��11�֣����ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺
��ȡWg���ᾧ�壬���100.00mL��ɫˮ��Һ����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L-1��KMnO4��Һ�ζ����Իش�
��1���ζ�ʱ��������Ӧ�Ļ�ѧ����ʽΪ
��2�������Ƕ�Ԫ���ᣬ�����ĵ��뷽��ʽΪ ��ӡ�����ƽ�⡱�ĽǶȽ��ͣ�Ϊʲô����ĵڶ�������ȵ�һ����
ͼI��ʾ100mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����10mL������̶�AΪ30����Ͳ��Һ��������___________mL��ͼII��ʾ25mL�ζ�����Һ���λ�ã����Һ�洦�Ķ�����a����ζ�����Һ������������ţ�_____________��
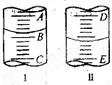
A.��amL B.��(25-a)mL
C.һ������amL D.һ������(25-a)mL
��3��ʵ���У���ҺKMnO4��ҺӦװ��_______ʽ�ζ����С����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ����ζ����յ㣬������õ�xֵ��_____��ƫ��ƫС����Ӱ�죩�ﵽ�ζ��յ㣬��Һ�� ɫ��Ϊ ɫ��
��4���ڵζ�����������amol��L-1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ_______mol��L-1;
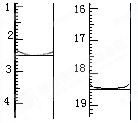
��5�������x=2����ȡij��ˮ�ϲ��ᾧ��0.1200 g��������ˮ��ȫ�ܽ⣬Ȼ����0.02000 mol��L-1������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ ��
(1) 2KMnO4 +5H2C2O4 + 3H2SO4 = K2SO4 + 10CO2�� +2MnSO4 + 8H2O
(2) H2C2O4 H++HC2O4����HC2O4��
H++HC2O4����HC2O4�� H++C2O42������һ��������������������Ƶڶ������� 22.0 D
H++C2O42������һ��������������������Ƶڶ������� 22.0 D
��3�� �� ��Ӱ�� ��ɫ �Ϻ�
��4��0.1aVmol/L
��5��84.00%
��������
�����������1��������غͲ�����Һ��Ӧ��ѧ����ʽΪ2KMnO4 +5H2C2O4 + 3H2SO4 = K2SO4 + 10CO2�� +2MnSO4 + 8H2O��
��2��������뷽��ʽΪ��H2C2O4 H++HC2O4����HC2O4��
H++HC2O4����HC2O4�� H++C2O42������һ��������������������Ƶڶ������롣
H++C2O42������һ��������������������Ƶڶ������롣
��Ͳ�̶���������������ÿһ���ʾ2.0mL����Ͳ��Һ������Ϊ22.0mL���ζ��̶ܿȴ��ϵ����������̶����滹�в���Һ�壬�ζ�����Һ������һ������(25��a)mL��
��3�����������Һ��ǿ�����ԣ�Ӧʢװ����ʽ�ζ����У����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ����ζ����յ㣬������õ�xֵ��Ӱ�죬�յ������ǵμ����һ�θ��������Һ����Һ����ɫ��Ϊ�Ϻ�ɫ��30s����ɫ��
��4��2KMnO4 �� 5H2C2O4
2 5
0.001avmol 0.025c
c=0.1avmol/L
��5�����ĸ��������Һ���Ϊ16.00mL������ᾧ�����ʵ���Ϊnmol��
5H2C2O4��2H2O��2 KMnO4
5 2
nmol 0.02��0.016mol
n=0.0008mol
H2C2O4��2H2O��������Ϊ84%��
���㣺���뷽��ʽ ��ѧʵ�� ������ԭ�ζ�
�������ø��������Һ�ζ���������������Һ��������ɫ���������ָʾ����


 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 180Va |
| W |
| 180Va |
| W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 50w |
| 9av |
| 50w |
| 9av |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ���ᾧ�����ɿ���H2C2O4?xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺
���ᾧ�����ɿ���H2C2O4?xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺| V |
| 10 |
| V |
| 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ��̶��ѧ������ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��16�֣��Ҷ��ᣨHOOC-COOH���������ᣬ������ˮ�����ڶ�Ԫ��ǿ�ᣬ�����Ϳ�ѧʵ�������Ź㷺����;�����ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺��ȡWg���ᾧ�壬���100.00mLˮ��Һ����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L-1��KMnO4��Һ�ζ���
��1�����������ķ�Ӧ����ʽ��ɲ���ƽ���������Ļ�ѧ����ʽд�ڴ���ϡ� KMnO4 + H2C2O4 + �� ��---K2SO4 + CO2��+ MnSO4 + �� ��
KMnO4 + H2C2O4 + �� ��---K2SO4 + CO2��+ MnSO4 + �� ��
��2������ʵ������в���Ҫ�������� ������ţ���
a��������ƽ�������룬���ӣ�b����ʽ�ζ��� c����ƿ
d��100mL����ƿ e���ձ� f��©�� g����ƿ
h�������� i��ҩ��
��3��ʵ���У���KMnO4��ҺӦʢװ��____ʽ�ζ����С��ζ��յ�ʱ��Һ��
��ɫ�仯Ϊ ��
��4���ڵζ�����������ȥamol��L-1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ________mol��L-1���ɴ˿ɼ���x��ֵ��___________��
���ú�W��a��V�Ĵ���ʽ��ʾ��
��5����С��ͬѧ��0.02mol���ᾧ�壨H2C2O4��2H2O�����뵽100mL0.2mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ����Һ�����ԣ������Һ�и�����Ũ���ɴ�С��˳��Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��09-10�꽭����������ѧ�߶���ѧ����ĩ���� ���ͣ�ʵ����
��13�֣������ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺
��ȡWg���ᾧ�壬���100.00mLˮ��Һ
��1����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L-1��KMnO4��Һ�ζ���KMnO4������ɫΪֹ���������ķ�Ӧ
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
�Իش𣺣�1��ʵ���в���Ҫ�������У�����ţ�___________����ȱ�ٵ������У������ƣ�___________________________________________________��
a.������ƽ�������룬���ӣ�b.�ζ��� c.100mL��Ͳ d.100mL����ƿ e.�ձ� f.©�� g.��ƿ h.������ i.ҩ�� j.��ƿ
��2��ʵ���У���ҺKMnO4��ҺӦװ��_____ʽ�ζ����У���Ϊ____ ___________��
��3�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ����ζ����յ㣬������õ�xֵ��__________________________��ƫ��ƫС����Ӱ�죩
��4���ڵζ�����������amol��L-1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ________________________mol��L-1���ɴ˿ɼ���x��ֵ��____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com