
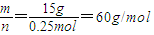 £¬£¬Ä¦¶ūÖŹĮæŌŚŹżÖµÉĻµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮ棬ĖłŅŌĻą¶Ō·Ö×ÓÖŹĮæĪŖ60£¬¹Ź“š°øĪŖ60£»
£¬£¬Ä¦¶ūÖŹĮæŌŚŹżÖµÉĻµČÓŚĘäĻą¶Ō·Ö×ÓÖŹĮ棬ĖłŅŌĻą¶Ō·Ö×ÓÖŹĮæĪŖ60£¬¹Ź“š°øĪŖ60£»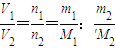 =
=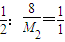 £¬ĖłŅŌM 2=16 g/mol
£¬ĖłŅŌM 2=16 g/mol 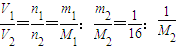 =
=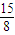 £¬ĖłŅŌM 2=30 g/mol
£¬ĖłŅŌM 2=30 g/mol 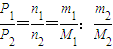 =
=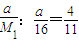 £¬ĖłŅŌM1=44 g/mol
£¬ĖłŅŌM1=44 g/mol 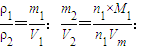
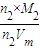 =
=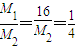 £¬
£¬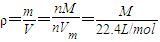 =3.170g/L ĖłŅŌM=71 g/mol£¬¹Ź“š°øĪŖ71 g/mol£¬
=3.170g/L ĖłŅŌM=71 g/mol£¬¹Ź“š°øĪŖ71 g/mol£¬


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
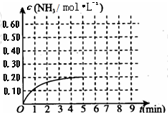 £Ø2010?ŃĢĢØŅ»Ä££©Ä³ĪĀ¶ČŹ±£¬ŌŚŅ»ČŻ»żĪŖ1LµÄĆܱÕČŻĘ÷ÖŠ£¬¼ÓČė0.4molµÄN2ŗĶ1.2molµÄH2£¬ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśČēĻĀ·“Ó¦£ŗN2£Øg£©+3H2£Øg£©?2NH3£Øg£©”÷H£¼0£¬5minŹ±“ļµ½Ę½ŗā£¬·“Ó¦ÖŠNH3µÄĪļÖŹµÄĮæÅØ¶ČµÄ±ä»ÆĒéæöČēĶ¼ĖłŹ¾£ŗĒė»Ų“šĻĀĮŠĪŹĢā£ŗ£Ø1£©øł¾ŻÉĻĶ¼£¬¼ĘĖć“Ó·“Ó¦æŖŹ¼µ½Ę½ŗāŹ±£¬Ę½¾ł·“Ó¦ĖŁĀŹv£ØN2£©=
£Ø2010?ŃĢĢØŅ»Ä££©Ä³ĪĀ¶ČŹ±£¬ŌŚŅ»ČŻ»żĪŖ1LµÄĆܱÕČŻĘ÷ÖŠ£¬¼ÓČė0.4molµÄN2ŗĶ1.2molµÄH2£¬ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśČēĻĀ·“Ó¦£ŗN2£Øg£©+3H2£Øg£©?2NH3£Øg£©”÷H£¼0£¬5minŹ±“ļµ½Ę½ŗā£¬·“Ó¦ÖŠNH3µÄĪļÖŹµÄĮæÅØ¶ČµÄ±ä»ÆĒéæöČēĶ¼ĖłŹ¾£ŗĒė»Ų“šĻĀĮŠĪŹĢā£ŗ£Ø1£©øł¾ŻÉĻĶ¼£¬¼ĘĖć“Ó·“Ó¦æŖŹ¼µ½Ę½ŗāŹ±£¬Ę½¾ł·“Ó¦ĖŁĀŹv£ØN2£©=| c(NH3)2 |
| c(N2)£®c(H2)3 |
| c(NH3)2 |
| c(N2)£®c(H2)3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓƶčŠŌµē¼«µē½ā2L1.0mol”¤L-1CuSO4ČÜŅŗ£¬ŌŚµēĀ·ÖŠĶعż0.5molµē×Óŗ󣬵÷»»Õżøŗ¼«¼ĢŠųµē½ā£¬µēĀ·ÖŠÓÖĶعżĮĖ1mo1µē×Ó£¬“ĖŹ±ČÜŅŗÖŠc(H+)(¼ŁÉčČÜŅŗĢå»ż²»±ä)ĪŖ£Ø £©
A.1.5mol”¤L-1 B.0.75mol”¤L-1
C.0.5mol”¤L-1 D.0.25mol”¤L-1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ±±¾©ŹŠøßČżÉĻѧʌæŖѧ²āŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŃ”ŌńĢā
ÓƶčŠŌµē¼«µē½ā2L1mol•L£1µÄCuSO4ČÜŅŗ£¬ŌŚµēĀ·ÖŠĶعż0.5molµē×Óŗ󣬵÷»»µēŌ“µÄÕżøŗµē¼«£¬µēĀ·ÖŠÓÖĶعż0.1molµē×Ó£¬“ĖŹ±ČÜŅŗÖŠµÄc(H£«)ŹĒ£Ø¼ŁÉčĶصēŗóČÜŅŗµÄĢå»ż²»±ä£©
A. 0.2mol•L£1 B. 0.6mol•L£1 C. 0.5mol•L£1 D. 0.25mol•L£1
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com