
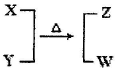
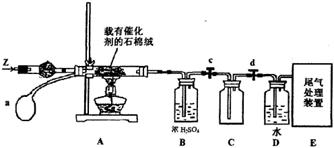
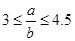 ����ԭ��HNO3������Ϊ g��
����ԭ��HNO3������Ϊ g�� 2NH3(g) ��H="-92.4" kJ��mol
2NH3(g) ��H="-92.4" kJ��mol 2NH3��+2H2O+CaCl2��NH3��H2O
2NH3��+2H2O+CaCl2��NH3��H2O NH3��+H2O
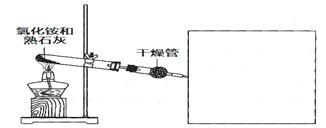
NH3��+H2O 4NO+6H2O ������H2O��g����δ��Ӧ��NH3
4NO+6H2O ������H2O��g����δ��Ӧ��NH3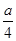
 2NH3(g) ��H="-92.4" kJ��mol
2NH3(g) ��H="-92.4" kJ��mol Fe(NO3)3��NO��2H2O
Fe(NO3)3��NO��2H2O 3Fe(NO3)2��2NO��4H2O
3Fe(NO3)2��2NO��4H2O 3Fe(NO3)2��Fe(NO3)3��3NO��6H2O
3Fe(NO3)2��Fe(NO3)3��3NO��6H2O

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͭ��Ũ���ᷴӦ | B��ͭ��ϡ���ᷴӦ |
| C������ͭ�����ᷴӦ | D������ͭ����������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1���NO | B��2���NO2��0.5���O2 |
| C��2���O2 | D��0.25���O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��N2��O2��Ӧ����NO |
| B��NH3������������NO |
| C��N2��H2��һ�������ºϳɰ� |
| D������ֲ��ĸ������������еĵ���ת��Ϊ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
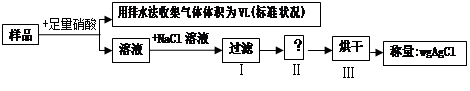
| A���ռ�����V L����Ϊ NO |
| B����ȱ�ٲ����ᵼ��ʵ����ƫС |
C����ͭ���Ͻ�ԭ���������Ϊ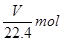 |
| D������m��V����ȷ��ͭ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ����O2��NO2�Ļ������ | B��������NO��NO2�Ļ������ |
| C��������N2��NO2�Ļ������ | D��ֻ������NO2һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ռӦ���Ȳ������� | B����������ˮ |
| C�����ȷֽⶼ�������� | D�����Ǿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ϳɰ���ҵ�н������������������ɰ��� |
| B��N2��O2���ŵ�����NO |
| C��ͨ����ѹ�����µȷ���������ת��ΪҺ̬�� |
| D��NH3��H2SO4������������(NH4)2SO4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com