
����20�֣�
(i) ���з�����Ϊ58�ļ����л����д�������������л���Ľṹ��ʽ
��1�������л���Ϊ��������ܵĽṹ��ʽΪ�� �� ��
��2�������л�����һ�ֱ���һԪȩ������ṹ��ʽΪ�� ��
��3�������л���1mol��������������Һ���ÿ�����4molAg�����л���Ľṹ��ʽΪ�� ��
��4�������л������������Ʒ�Ӧ�ų�����������ʹ������Ȼ�̼��Һ��ɫ������л���ṹ��ʽ������ ����ע���ǻ�����˫���ϵ��л��K���ȶ���
��ii����֪���� R-CH2-COOH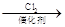
 ��
��
�� R-ONa R-O-R�� (R-��R��-��������)��
R-O-R�� (R-��R��-��������)��
��֪ij������һ�־�������������ʳ�����ϣ�������·�ߣ���Ӧ������ȥ�����£�
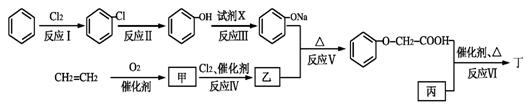
��ش��������⣺
��1����������Է�������Ϊ58��1 mol����ȫȼ�տɲ���3.0 mol CO2��3.0 mol H2O���ұ������в�������Ϊ��״�ṹ������ṹ��ʽ�� ��
��2����Ӧ��������� ����Ӧ���ķ�Ӧ������ ��
��3���ҵĽṹ��ʽ�� ��
��Ӧ���Ļ�ѧ����ʽ�� ��
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����20�֣�
(i) ���з�����Ϊ58�ļ����л����д�������������л���Ľṹ��ʽ
��1�������л���Ϊ��������ܵĽṹ��ʽΪ�� �� ��
��2�������л�����һ�ֱ���һԪȩ������ṹ��ʽΪ�� ��
��3�������л���1mol��������������Һ���ÿ�����4molAg�����л���Ľṹ��ʽΪ�� ��
��4�������л������������Ʒ�Ӧ�ų�����������ʹ������Ȼ�̼��Һ��ɫ������л���ṹ��ʽ������ ����ע���ǻ�����˫���ϵ��л��K���ȶ���
��ii����֪���� R-CH2-COOH
��
�� R-ONaR-O-R�� (R-��R��-��������)��
��֪ij������һ�־�������������ʳ�����ϣ�������·�ߣ���Ӧ������ȥ�����£�
��ش��������⣺
��1����������Է�������Ϊ58��1mol����ȫȼ�տɲ���3.0 mol CO2��3.0 mol H2O���ұ������в�������Ϊ��״�ṹ������ṹ��ʽ�� ��
��2����Ӧ��������� ����Ӧ���ķ�Ӧ������ ��
��3���ҵĽṹ��ʽ�� ��
��Ӧ���Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡɽ���и�һ2���¿���ѧ�Ծ� ���ͣ������
����6�֣�A��B��C��D�ǰ�ԭ��������С�������е�ǰ20��Ԫ�صĵ��ʡ�B��E��Ϊ��ɿ����ijɷ֡�F����ɫ��Ӧ�ʻ�ɫ����G�У��ǽ���Ԫ�������Ԫ�ص�ԭ�Ӹ�����Ϊ1��2����һ�������£�������֮����ת����ϵ����(���ֲ���δ�г�)��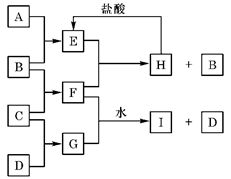
��1��A�� ��C�� ��
��2��H�����ᷴӦ����E�Ļ�ѧ����ʽ�� ��
��3��E��F��Ӧ�Ļ�ѧ����ʽ��
��
��4��F��G��ˮ��Һ��Ӧ����I��D�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ����ɽ���������ѧ����ĩ��1�£����Ի�ѧ�Ծ��������棩 ���ͣ������
�����12�֣�
��þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO��ռ40%��������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ��Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO4��7H2O���������£�

��������ش�
1��ʵ��������0.8mol/L������800 mL������98%��Ũ���ᣨ��= 1.84 g/mL�������ƣ���ȡŨ����ʱ����ʹ�õ���Ͳ�Ĺ��Ϊ
A��10 mL B��20 mL C��50 mL D��100 mL
2�������NaClO����Mn2+��Ӧ��Mn2+ + ClO- + H2O �� MnO2�� + 2H+ + Cl-������һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ ��
3����������Ҫ�ɷݳ�����Fe(OH)3��Al(OH)3�⣬���� ��
4���ڡ����ơ�ǰ���������Һ��Fe3+�Ƿ������������鷽�� ��
5����֪MgSO4��CaSO4���ܽ�����±���
|
�¶ȣ��棩 |
40 |
50 |
60 |
70 |
|
MgSO4 |
30.9 |
33.4 |
35.6 |
36.9 |
|
CaSO4 |
0.210 |
0.207 |
0.201 |
0.193 |
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵���������� ��������I���ǽ���Һ��������Ũ������ȴ�ᾧ�� ����õ���MgSO4��7H2O��
6��ʵ�����ṩ����þ�100 g���õ���MgSO4��7H2OΪ172.2 g����MgSO4��7H2O�IJ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ���Ի�ѧ���⣨���Ͼ��� ���ͣ������
(20��)
18-I(6��)��֪�� �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����
A. 2-��-l��3-����ϩ��2-��Ȳ B��1��3-���ϩ��2-��Ȳ
C��2��3-����-1��3-���ϩ����Ȳ D��2��3-����-l��3-����ϩ�ͱ�Ȳ
18-II(14��)A��G�����л���������ǵ�ת����ϵ���£�

��ش��������⣺
(1)��֪��6.0g������E��ȫȼ������8.8g C02��3.6g H20��E������������������ܶ�Ϊ30����E�ķ���ʽΪ_______________��[��Դ:ZXXK]
(2)AΪһȡ��������B�к���һ��������B����C�Ļ�ѧ����ʽΪ_______________��
(3)��B����D����C����D�ķ�Ӧ�����ֱ���_______________��_______________��
(4)��A����B����D����G�ķ�Ӧ���ͷֱ���_______________��_______________��
(5) F���������������У���ṹ��ʽΪ_______________��
(6)��G��ͬ���칹���У�������һ�����IJ���ֻ��һ�ֵĹ���___________�������к˴Ź�
������������壬�ҷ������Ϊl��1����_______________ (��ṹ��ʽ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com