
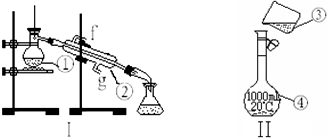
���� ��1�����������Ľṹ�ص㡢��;�ж������ƣ�
��2���л����Ͳ�����������ʹ��ʱ�������Ƿ�©ˮ��
��3����ȡ����ˮ�Ĺ��̱����þƾ��Ƽ��ȣ�ʵ����������̣��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
��4����������һ�����ʵ���Ũ�ȵ���Һ�ķ����Ͳ�����Ѱ��װ���еĴ���
��5������Ũ��Һ������ϡ��Һ��ʵ�����������������Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����
��6��������ƽ��ʹ�÷������������룬���̵������������̵����������������������Ʒ����=��������+�������������λ�÷ŷ����������̵�����=���̵�����+������������е�ʽ���м��㣻����240mL��Һ����Ҫѡ��250mL����ƿ������250mL 1mol/L������������Һ���������Ƶ����ʵ���������������Ƶ�������
��7������c=$\frac{n}{V}$�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��� �⣺��1����Ϊ���ڷ���е����ϴ������Һ��ķ�����ѻӷ��Թ�����Һ��ķ����������Ϊ������ƿ����Ϊ��ȴ����ΪҺ��ij������������ܣ�
�ʴ�Ϊ��������ƿ�������ܣ�
��2����Ϊ�ձ�����Ϊ����ƿ������ƿ��ʹ��ʱ��ҡ�ȣ�����ʹ��ǰҪ����Ƿ�©ˮ��������ƿ�������ܲ���Ҫ����Ƿ�©ˮ��
�ʴ�Ϊ���ܣ�
��3����ȡ����ˮ��ʵ����������̣������þƾ��ƣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ�
�ʴ�Ϊ���ƾ��ƣ�g��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ��
�ʴ�Ϊ��δ�ò�����������δ����250ml����ƿ��
��5������һ�����ʵ���Ũ�ȵ���Һ����Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ�IJ���˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
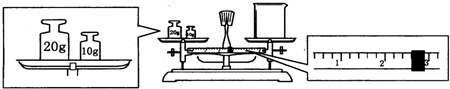
��6�������̵�����=���̵�����+�����������֪����������=�ձ�����+����������������ձ�����=��������-�������������ձ�����=20g+10g-2.6g=27.4g��ʵ����û��240mL����ƿ��ʵ�������Ƶ���250mL 1mol/L������������Һ����Ҫ�������Ƶ����ʵ���Ϊ��1mol/L��0.25L=0.25mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.25mol=10.0g��
�ʴ�Ϊ��27.4��10.0g��
��7����û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʢٲ�ѡ��
��ת����Һʱ������������Һ��������ƿ���棬�������ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ��ʢڲ�ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ��䣬�ʢ۲�ѡ��
�ܶ���ʱ���ӿ̶��ߣ�������Һ�����ƫС����Һ��Ũ��ƫ�ߣ��ʢ�ѡ��
�ݶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ��ʢݲ�ѡ��
��ѡ���ܣ�
���� ���⿼��������һ�����ʵ���Ũ����Һ�����ƣ���Ϥʵ��ԭ����������ʹ�÷����ǽ���ؼ�����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
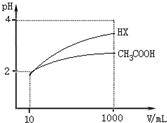 25��ʱ��������ĵ���ƽ�ⳣ�����£�
25��ʱ��������ĵ���ƽ�ⳣ�����£�| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10-5 | K1 4.3��10-7 K2 5.6��10-11 | 3.0��10-8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����NaOH��Һʱ���������õ�NaOH�������С�ձ����ܽ⣬δ����ȴ����ת�Ƶ�����ƿ�в����� | |
| B�� | ת�Ƶ�����ƿ�����У���������Һ���� | |
| C�� | ת�ƺ�δϴ��С�ձ��Ͳ����� | |
| D�� | ����ʱ���ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ǰ��ϡ������ϴ����ƿ��δ������ˮϴ�� | |
| B�� | ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��ټ�������ˮ����̶������� | |
| C�� | ϴ����Ͳ������ϴ��Һת������ƿ | |
| D�� | ����ʱ���Ӷ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
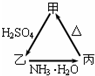 �ס��ҡ�����������֮��������ͼ��ʾ��ת����ϵ��
�ס��ҡ�����������֮��������ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com