
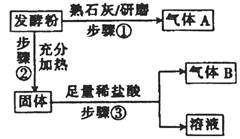
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ��������������� ����Һ�ֳ����ݣ��ֱ�װ��A��B�Թ��С� | |
| ����2��_____________________________ __________________________________ | ________________________֤����Na+���� �ͷ�����NaHCO3�� |
| ����3��_____________________________ ___________________________________ | ___________________________________ _______����ϲ���2�еĽ��ۣ�����2������ |
| ����2���ýྻ�IJ�˿պȡA�е���Һ���� �ƾ������������գ��۲���ɫ��2�֣� | ��ɫ�ʻ�ɫ��1�֣� |
| ����3��������1����B�Թ�����εμ� 0.1 mol/L NaOH��Һ��2�֣� | �����а�ɫ�������ɣ����Ȳ�����ɫ����������ܽ⣩��1�֣���֤�����ͷ�������������1�֣� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��A1C13���ܴ��� | B��������Һ����ɫ |
| C��Ag (NH3)2+����������� | D��������Һ��c (OH��)��c (Cl��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ��Һ��һ������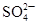 |
| B | ��ij��Һ�м������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ���� | ��Һ��һ������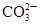 |
| C | ����ȼƲⶨSO2��CO2������Һ��pH��ǰ��pHС | H2SO3����ǿ��H2CO3 |
| D | �ò�����պȡŨ��ˮ�㵽��ɫʯ����ֽ�ϣ���ֽ����ɫ | Ũ��ˮ�ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | ��������� |
| ����1 �����Թ��м��������������ټ�������_________________������� | ����Һ��ɫ���Ըı䣬����_______���ɣ���֤���������ʴ��� |
| ����2�� ������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȡ����2�õ��������������Թ��У� �μ�___________________________________ _______________________________________ | __________________________________ ___________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
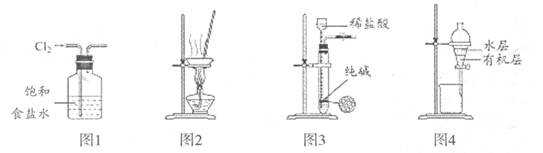
| A����ͼ1װ�ó�ȥC12��������HCl |
| B����ͼ2װ������NH4Cl������Һ��ȡNH4Cl���� |
| C����ͼ3װ����ȡ������CO2���� |
| D����ͼ4װ�÷���CCl4��ȡ��ˮ����л����ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| A��BaCl2 | B��BaCO3 | C��Ba(NO3)2 | D��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
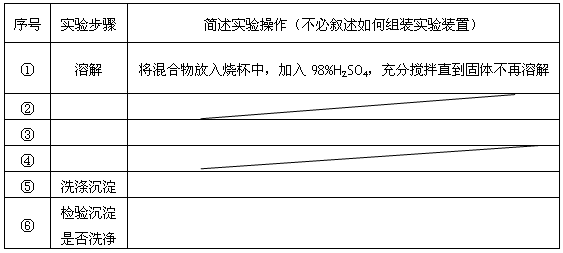
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com