
ĖįŠŌĢõ¼žĻĀ£¬ĪżŌŚĖ®ČÜŅŗÖŠÓŠSn2+”¢Sn4+Į½ÖÖÖ÷ŅŖ“ęŌŚŠĪŹ½”£SnSO4ŹĒŅ»ÖÖÖŲŅŖµÄĮņĖįŃĪ£¬¹ć·ŗÓ¦ÓĆÓŚ¶ĘĪż¹¤Ņµ£¬ĘäÖʱøµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© SnCl2ÓĆŃĪĖį¶ų²»ÓĆĖ®Ö±½ÓČܽāµÄŌŅņŹĒ £¬¼ÓČėĪż·ŪµÄ×÷ÓĆŹĒ ”£
£Ø2£© ·“Ó¦IÉś³ÉµÄ³ĮµķĪŖSnO£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
ӣ
£Ø3£© ¼ģŃé³ĮµķŅŃ¾”°Ļ“µÓ”±øɾ»µÄ²Ł×÷ŹĒ£ŗ ”£
£Ø4£©·“Ó¦IIĮņĖįµÄ×÷ÓĆÖ®Ņ»ŹĒæŲÖĘČÜŅŗµÄpH”£ČōČÜŅŗÖŠc(Sn2+)=1.0mol”¤L£1£¬ŌņŹŅĪĀĻĀÓ¦æŲÖĘČÜŅŗpH ”££ØŅŃÖŖ£ŗKsp[Sn(OH)2]=1.0”Į10£26£©
£Ø5£©ĖįŠŌĢõ¼žĻĀ£¬SnSO4»¹æÉÓĆ×÷Ė«ŃõĖ®µÄČ„³ż¼Į£¬ŹŌŠ“³öĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ӣ
£Ø6£©³±ŹŖ»·¾³ÖŠ£¬¶ĘĪżĶ¼“Ź¹Īż²ćĘĘĖšŅ²ÄÜ·ĄÖ¹ŠĪ³ÉĶĀĢ£¬Ēė½įŗĻÓŠ¹ŲµÄŌĄķ½āŹĶĘäŌŅņ£ŗ ”£
£Ø16·Ö£©
£Ø1£©ŅÖÖĘSn2+Ė®½ā£Ø2·Ö£© ·ĄÖ¹Sn2+±»Ńõ»Æ£Ø2·Ö£©
£Ø2£©SnCl2 + Na2CO3 =" SnO”ż" + CO2”ü+2NaCl£Ø2·Ö£©
£Ø3£©Č”ÉŁĮæ×īŗóŅ»“ĪĻ“µÓŅŗÓŚŹŌ¹ÜÖŠ£¬µĪČė¼øµĪAgNO3ČÜŅŗ£¬ČōĪŽ°×É«³ĮµķÉś³É£¬ŌņĖµĆ÷³ĮµķŅŃĻ“µÓøɾ»”££Ø3·Ö£©
£Ø4£©Š”ÓŚ1 £Ø3·Ö”£ČōŠ“³É”Ü1øų1·Ö£©
£Ø5£©Sn2+ + H2O2 + 2H+ = Sn4+ + 2H2O£Ø2·Ö£©
£Ø6£©³±ŹŖ»·¾³ÖŠ£¬SnÓėCu¹¹³ÉŌµē³Ų£¬Sn×÷ĪŖøŗ¼«£¬±£»¤Õż¼«Cu²»±»Ńõ»Æ”££Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©SnCl2ŹĒĒæĖįČõ¼īŃĪ£¬ÄÜĖ®½ā£¬¼“SnCl2+2H2O Sn(OH)2+2HCl£¬Ö±½ÓÓĆĖ®Čܽā»ņĻ”ŹĶÄÜ“Ł½ųSn2+Ė®½ā£¬ÓĆŃĪĖįČܽā£¬Ōö“óĒāĄė×Ó»ņĀČ»ÆĒāÅØ¶ČŹ¹Ė®½āĘ½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬¼“ŅÖÖĘSn2+Ė®½ā£»¶ĮĶ¼æÉÖŖ£¬Äæ±ź²śĪļŹĒSnSO4£¬ĘäÖŠĖłŗ¬ĪżŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+2¼Ū£¬ŅŃÖŖĪżŌŚĖ®ČÜŅŗÖŠÓŠSn2+”¢Sn4+£¬ĖµĆ÷¼ÓČėĪż·ŪµÄÖ÷ŅŖÄæµÄŹĒ·ĄÖ¹Sn2+Ńõ»ÆĪŖSn4+»ņÕß½«Sn4+»¹ŌĪŖSn2+£»£Ø2£©Na2CO3ÓėHClČŻŅ×·¢Éś·“Ó¦£¬Éś³ÉNaCl”¢H2OŗĶCO2ĘųĢ壬“Ł½ųSnCl2Ė®½ā£¬Éś³ÉµÄSn(OH)2Ņ×·Ö½āĪŖSnO³ĮµķŗĶĖ®£¬øł¾Ż¹¤ŅÕĮ÷³ĢĶ¼Ė³ĶĘŗĶÄęĶĘ£¬·“Ó¦IµÄ·“Ó¦ĪļĪŖSnCl2”¢Na2CO3£¬Éś³ÉĪļĪŖSnO”¢NaCl”¢CO2£¬ÓÉӌƻӊŌŖĖŲ»ÆŗĻ¼Ū±ä»Æ£¬Ōņ·“Ó¦IŹōÓŚø“·Ö½ā·“Ó¦£¬øł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬ŌņSnCl2+Na2CO3=SnO”ż+CO2”ü+2NaCl£»£Ø3£©ÓÉÓŚ³Įµķ¾ßÓŠ½ĻĒæµÄĪüø½ŠŌ£¬ŌņSnO³ĮµķÖŠĪüø½ĮĖæÉČÜŠŌµÄNaClŗĶ¹żĮæµÄNa2CO3£¬Ņņ“Ė¼ģŃé³ĮµķŅŃ¾Ļ“µÓøɾ»µÄ·½·ØŹĒÉč¼ĘŃęÉ«·“Ó¦²»ŹĒ»ĘÉ«»ņ¼ģŃéĀČĄė×Ó»ņĢ¼ĖįøłĄė×ӵďµŃé·½°ø£¬×ī¼ņµ„ŗĻĄķµÄ²Ł×÷ŹĒȔɣĮæ×īŗóŅ»“ĪĻ“µÓŅŗÓŚŹŌ¹ÜÖŠ£¬µĪČė¼øµĪAgNO3ČÜŅŗ£¬ČōĪŽ°×É«³ĮµķÉś³É£¬ŌņĖµĆ÷³ĮµķŅŃĻ“µÓøɾ»£»£Ø4£©ÓÉÓŚSn(OH)2(s)
Sn(OH)2+2HCl£¬Ö±½ÓÓĆĖ®Čܽā»ņĻ”ŹĶÄÜ“Ł½ųSn2+Ė®½ā£¬ÓĆŃĪĖįČܽā£¬Ōö“óĒāĄė×Ó»ņĀČ»ÆĒāÅØ¶ČŹ¹Ė®½āĘ½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬¼“ŅÖÖĘSn2+Ė®½ā£»¶ĮĶ¼æÉÖŖ£¬Äæ±ź²śĪļŹĒSnSO4£¬ĘäÖŠĖłŗ¬ĪżŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+2¼Ū£¬ŅŃÖŖĪżŌŚĖ®ČÜŅŗÖŠÓŠSn2+”¢Sn4+£¬ĖµĆ÷¼ÓČėĪż·ŪµÄÖ÷ŅŖÄæµÄŹĒ·ĄÖ¹Sn2+Ńõ»ÆĪŖSn4+»ņÕß½«Sn4+»¹ŌĪŖSn2+£»£Ø2£©Na2CO3ÓėHClČŻŅ×·¢Éś·“Ó¦£¬Éś³ÉNaCl”¢H2OŗĶCO2ĘųĢ壬“Ł½ųSnCl2Ė®½ā£¬Éś³ÉµÄSn(OH)2Ņ×·Ö½āĪŖSnO³ĮµķŗĶĖ®£¬øł¾Ż¹¤ŅÕĮ÷³ĢĶ¼Ė³ĶĘŗĶÄęĶĘ£¬·“Ó¦IµÄ·“Ó¦ĪļĪŖSnCl2”¢Na2CO3£¬Éś³ÉĪļĪŖSnO”¢NaCl”¢CO2£¬ÓÉӌƻӊŌŖĖŲ»ÆŗĻ¼Ū±ä»Æ£¬Ōņ·“Ó¦IŹōÓŚø“·Ö½ā·“Ó¦£¬øł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬ŌņSnCl2+Na2CO3=SnO”ż+CO2”ü+2NaCl£»£Ø3£©ÓÉÓŚ³Įµķ¾ßÓŠ½ĻĒæµÄĪüø½ŠŌ£¬ŌņSnO³ĮµķÖŠĪüø½ĮĖæÉČÜŠŌµÄNaClŗĶ¹żĮæµÄNa2CO3£¬Ņņ“Ė¼ģŃé³ĮµķŅŃ¾Ļ“µÓøɾ»µÄ·½·ØŹĒÉč¼ĘŃęÉ«·“Ó¦²»ŹĒ»ĘÉ«»ņ¼ģŃéĀČĄė×Ó»ņĢ¼ĖįøłĄė×ӵďµŃé·½°ø£¬×ī¼ņµ„ŗĻĄķµÄ²Ł×÷ŹĒȔɣĮæ×īŗóŅ»“ĪĻ“µÓŅŗÓŚŹŌ¹ÜÖŠ£¬µĪČė¼øµĪAgNO3ČÜŅŗ£¬ČōĪŽ°×É«³ĮµķÉś³É£¬ŌņĖµĆ÷³ĮµķŅŃĻ“µÓøɾ»£»£Ø4£©ÓÉÓŚSn(OH)2(s) Sn2++2OH££¬ŌņKsp[Sn(OH)2]=c(Sn2+)?c2(OH£)£¬¼“c2(OH£)= Ksp[Sn(OH)2]/c(Sn2+)£¬c(OH£)= 1.0”Į10£13mol/L£»ÓÉÓŚKw= c(H+)?c(OH£)= 1.0”Į10£14£¬Ōņc(H+)= Kw/c(OH£)= 1.0”Į10£1 mol/L£¬ŌņpH="”Ŗlg" c(H+)=1£»ĪŖĮĖ·ĄÖ¹Īö³öSn(OH)2³Įµķ£¬±ŲŠėŹ¹ČÜŅŗÖŠQc[Sn(OH)2]<Ksp[Sn(OH)2]£¬Ōņc(OH£) <1.0”Į10£13mol/L£¬¼“c(H+)>1.0”Į10£1 mol/L£¬pH<1£»£Ø5£©Ė«ŃõĖ®¾ßÓŠŃõ»ÆŠŌ£¬Äܽ«ĪżŌŖĖŲ“Ó+2¼ŪŃõ»ÆĪŖ+4¼Ū£¬¶ų¹żŃõ»ÆĒā±»»¹ŌĪŖĖ®£¬øł¾Ż×īŠ”¹«±¶Źż·ØÅäĘ½£¬Óɵē×Ó”¢µēŗÉŗĶŌ×ÓŹŲŗćæÉµĆ£ŗSn2+ + H2O2 + 2H+ = Sn4+ + 2H2O£»£Ø6£©½šŹō»ī¶ÆĖ³Šņ±ķÖŠĪżĪ»ÓŚĶĒ°£¬¹¹³ÉŌµē³ŲŹ±£¬ĪżŹĒøŗ¼«£¬ĶŹĒÕż¼«£¬ŌņĪż±»øÆŹ“£¬Ķ±»±£»¤£¬¼“Ź¹Īż²ćĘĘĖšŅ²ÄÜ·ĄÖ¹ĶøÆŹ“£¬ÕāŹĒĪžÉüŃō¼«µÄŅõ¼«±£»¤·ØµÄÓ¦ÓĆ”£
Sn2++2OH££¬ŌņKsp[Sn(OH)2]=c(Sn2+)?c2(OH£)£¬¼“c2(OH£)= Ksp[Sn(OH)2]/c(Sn2+)£¬c(OH£)= 1.0”Į10£13mol/L£»ÓÉÓŚKw= c(H+)?c(OH£)= 1.0”Į10£14£¬Ōņc(H+)= Kw/c(OH£)= 1.0”Į10£1 mol/L£¬ŌņpH="”Ŗlg" c(H+)=1£»ĪŖĮĖ·ĄÖ¹Īö³öSn(OH)2³Įµķ£¬±ŲŠėŹ¹ČÜŅŗÖŠQc[Sn(OH)2]<Ksp[Sn(OH)2]£¬Ōņc(OH£) <1.0”Į10£13mol/L£¬¼“c(H+)>1.0”Į10£1 mol/L£¬pH<1£»£Ø5£©Ė«ŃõĖ®¾ßÓŠŃõ»ÆŠŌ£¬Äܽ«ĪżŌŖĖŲ“Ó+2¼ŪŃõ»ÆĪŖ+4¼Ū£¬¶ų¹żŃõ»ÆĒā±»»¹ŌĪŖĖ®£¬øł¾Ż×īŠ”¹«±¶Źż·ØÅäĘ½£¬Óɵē×Ó”¢µēŗÉŗĶŌ×ÓŹŲŗćæÉµĆ£ŗSn2+ + H2O2 + 2H+ = Sn4+ + 2H2O£»£Ø6£©½šŹō»ī¶ÆĖ³Šņ±ķÖŠĪżĪ»ÓŚĶĒ°£¬¹¹³ÉŌµē³ŲŹ±£¬ĪżŹĒøŗ¼«£¬ĶŹĒÕż¼«£¬ŌņĪż±»øÆŹ“£¬Ķ±»±£»¤£¬¼“Ź¹Īż²ćĘĘĖšŅ²ÄÜ·ĄÖ¹ĶøÆŹ“£¬ÕāŹĒĪžÉüŃō¼«µÄŅõ¼«±£»¤·ØµÄÓ¦ÓĆ”£
æ¼µć£ŗæ¼²éÓŠ¹ŲĪļÖŹÖʱøµÄ»Æѧ¹¤ŅÕĮ÷³Ģ£¬Éę¼°½āŹĶ¼ÓČėŹŌ¼ĮµÄŌŅņŗĶ×÷ÓĆ”¢ŹéŠ“¹Ų¼ü²½ÖčµÄ»Æѧ·½³ĢŹ½”¢Éč¼Ę¼ģŃé³ĮµķŹĒ·ńĻ“µÓøɾ»µÄŹµŃé·½°ø”¢ČܶȻżŗĶĖ®µÄĄė×Ó»żµÄÓŠ¹Ų¼ĘĖć”¢ŹéŠ“Ö÷ŅŖĮ÷³ĢµÄĄė×Ó·½³ĢŹ½”¢øł¾ŻŌµē³ŲŌĄķ½āŹĶĪż²ćĘĘĖšŅ²ÄÜ·ĄÖ¹ĶøÆŹ“µÄŌŅņµČ”£


 ĆūĢā½š¾ķĻµĮŠ“š°ø
ĆūĢā½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)
LiPF6ŹĒļ®Ąė×Óµē³ŲÖŠ¹ć·ŗÓ¦ÓƵĵē½āÖŹ”£Ä³¹¤³§ÓĆLiF”¢PCl5ĪŖŌĮĻ£¬µĶĪĀ·“Ó¦ÖʱøLiPF6£¬ĘäĮ÷³ĢČēĻĀ£ŗ
ŅŃÖŖ£ŗHClµÄ·ŠµćŹĒ£85.0 ”ę£¬HFµÄ·ŠµćŹĒ19.5 ”ę”£
£Ø1£©µŚ¢Ł²½·“Ó¦ÖŠĪŽĖ®HFµÄ×÷ÓĆŹĒ ”¢ ”£·“Ó¦Éč±ø²»ÄÜÓĆ²£Į§²ÄÖŹµÄŌŅņŹĒ (ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)”£ĪŽĖ®HFÓŠøÆŹ“ŠŌŗĶ¶¾ŠŌ£¬¹¤³§°²Č«ŹÖ²įĢįŹ¾£ŗČē¹ū²»Š”ŠÄ½«HFÕ“µ½Ę¤·ōÉĻ£¬æÉĮ¢¼“ÓĆ2%µÄ ČÜŅŗ³åĻ“”£
£Ø2£©øĆĮ÷³ĢŠčŌŚĪŽĖ®Ģõ¼žĻĀ½ųŠŠ£¬µŚ¢Ū²½·“Ó¦ÖŠPCl5¼«Ņ×Ė®½ā£¬Ęä²śĪļĪŖĮ½ÖÖĖį£¬Š“³öPCl5Ė®½āµÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©µŚ¢Ü²½·ÖĄė²ÉÓƵķ½·ØŹĒ £»µŚ¢Ż²½·ÖĄėĪ²ĘųÖŠHF”¢HCl²ÉÓƵķ½·ØŹĒ ”£
£Ø4£©LiPF6²śĘ·ÖŠĶس£»ģÓŠÉŁĮæLiF”£Č”ѳʷwg”£²āµĆLiµÄĪļÖŹµÄĮæĪŖnmol£¬ŌņøĆѳʷ֊LiPF6µÄĪļÖŹµÄĮæĪŖ mol(ÓĆŗ¬ÓŠw”¢nµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓĆĆ¢Ļõ£ØNa2SO4 ”ć10H2O£©Öʱø“æ¼ī”¢Ć÷·Æļ§[£ØNH4£©2Al£ØSO4£©2”ć12H2O]µÄÉś²ś¹¤ŅÕĮ÷³ĢČēĻĀĶ¼£ŗ
£Ø1£©ČÜŅŗCÖŠµÄČÜÖŹÖ÷ŅŖŹĒ_____________
£Ø2£©Ć÷·Æļ§µÄČÜŅŗ³Ź_____ŠŌ£¬Ć÷·Æļ§æÉÓĆÓŚ¾»Ė®£¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ĘäŌĄķ______________________
£Ø3£©¹ż³Ģ¢ńÖŠµÄ·“Ó¦ĪĀ¶Č²»Äܳ¬¹ż40”ę£¬ĘäŌŅņŹĒ______________________________
£Ø4£©ŌĖÓĆ»ÆŃ§Ę½ŗāŌĄķ½āŹĶNa2SO4ÉŌ¹żĮæµÄŌŅņ
£Ø5£©Čō½«Al2£ØSO4£©3¼ÓČėµ½AÖŠ»į²śÉś“óĮæµÄ³ĮµķŗĶĘųĢå,µ¼ÖĀĆ÷·Æļ§µÄ²śĮæ½µµĶ,ĒėÓĆĄė×Ó·½³ĢŹ½½āŹĶ²śÉśøĆĻÖĻóµÄŌŅņŹĒ___________________________________________
£Ø6£©ČÜŅŗEÖŠµÄČÜÖŹĄė×ÓĪŖ__________________
£Ø7£©ŅŃÖŖĆ÷·Æļ§µÄČܽā¶ČĖę×ÅĪĀ¶ČµÄÉżø߶ųŌö“󣬹ż³Ģ¢ņÖŠµĆµ½Ć÷·Æļ§µÄĻµĮŠŹµŃé²Ł×÷ŹĒ: ”¢ ”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ņ»ÖÖŗ¬ĀĮ”¢ļ®”¢īܵĵē×Ó·ĻĮĻÖŠ£¬ĀĮŅŌĀĮ²µÄŠĪŹ½“ęŌŚ£¬īÜŅŌCo3O4µÄŠĪŹ½“ęŌŚ£ØĪüø½ŌŚĀĮ²µÄµ„Ćę»ņĖ«Ćę£©£¬ļ®»ģŌÓÓŚĘäÖŠ”£“ÓøĆ·ĻĮĻÖŠ»ŲŹÕCo3O4µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
£Ø1£©ČÜŅŗAµÄČÜÖŹµÄÖ÷ŅŖ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©
£Ø2£©īÜŌüÖŠ¼ÓČėĻ”H2SO4Ėį»Æŗó£¬ŌŁ¼ÓČėNa2S2O3ČÜŅŗæÉŅŌ½ž³öīÜĄė×Ó£¬Ōņ½ž³öīÜĄė×ӵĥė×Ó·½³ĢŹ½ĪŖ£Ø²śĪļÖŠÖ»ÓŠŅ»ÖÖĖįøł£© ”£
£Ø3£©ŌŚŹµŃéŹŅÄ£Äā¹¤ŅµÉś²śŹ±£¬Ņ²æÉÓĆŃĪĖį½ž³öīÜĄė×Ó£¬µ«Źµ¼Ź¹¤ŅµÉś²śÖŠ²»ÓĆŃĪĖį£¬Ēė“Ó·“Ó¦ŌĄķ·ÖĪö²»ÓĆŃĪĖį½ž³öīÜĄė×ÓµÄÖ÷ŅŖŌŅņ£ŗ ”£
£Ø4£©¼ÓČėNaFµÄ·“Ó¦ĪŖ£ŗLi£«£«F£ LiF”ż£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK= ”£
LiF”ż£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK= ”£
£Ø5£©¼ÓČė30%Na2CO3ČÜŅŗµÄ×÷ÓĆŹĒ ”£
£Ø6£©ŌŚæÕĘųÖŠ¶ĶÉÕCoCO3Éś³ÉCo3O4µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øõĖįĒ¦Ė׳Ęøõ»Ę£¬²»ČÜÓŚĖ®”£¹ć·ŗÓĆÓŚĶæĮĻ”¢ÓĶÄ«”¢Ęį²¼”¢ĖÜĮĻŗĶĪĽĢÓĆĘ·µČ¹¤Ņµ”£ŹµŃéŹŅÄ£Äā¹¤ŅµÉĻÓĆøõĪŪÄą£Øŗ¬ÓŠCr2O3”¢Fe2O3”¢A12O3”¢SiO2µČ£©Öʱøøõ»ĘµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
£Ø1£©½«øõĪŪÄą·ŪĖéµÄÄæµÄŹĒ £¬²Ł×÷aµÄĆū³ĘĪŖ £»
£Ø2£©·ĻŌüµÄÖ÷ŅŖ³É·ÖŹĒA1(OH)3ŗĶFe(OH)3”£ŅŃÖŖ25”ꏱ£¬A1(OH)3µÄKsp=1.3”Į10£33£¬ŌņøĆĪĀ¶ČĻĀ·“Ó¦Al3++3H2O Al(OH)3+3H+µÄĘ½ŗā³£ŹżĪŖ £Ø±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö£©£»
Al(OH)3+3H+µÄĘ½ŗā³£ŹżĪŖ £Ø±£ĮōĮ½Ī»ÓŠŠ§Źż×Ö£©£»
£Ø3£©Š“³ö¼ÓČė30%H2O2¹ż³ĢÖŠ·¢ÉśµÄĄė×Ó·“Ó¦·½³ĢŹ½£ŗ £»
£Ø4£©ŹµŃéŹŅĻ“µÓøõ»Ę³ĮµķµÄ·½·Ø£ŗ £»
£Ø5£©Š“³öÅØŃĪĖįÓėA12O3·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
K2SO4ŹĒĪŽĀČÓÅÖŹ¼Ų·Ź£¬Mn3O4ŹĒÉś²śČķ“ÅĢśŃõĢå²ÄĮĻµÄÖ÷ŅŖŌĮĻ”£ŅŌĮņĖį¹¤ŅµµÄĪ²ĘųĮŖŗĻÖʱøK2SO4ŗĶMn3O4µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
£Ø1£©¼øÖÖŃĪµÄČܽā¶Č¼ūĶ¼”£·“Ó¦IIIÖŠ£¬Ļņ(NH4)2SO4ČÜŅŗÖŠ¼ÓČėKClČÜŅŗ³ä·Ö·“Ó¦ŗ󣬽ųŠŠÕō·¢ÅØĖõ”¢ ”¢Ļ“µÓ”¢øÉŌļµČ²Ł×÷¼“µĆK2SO4²śĘ·”£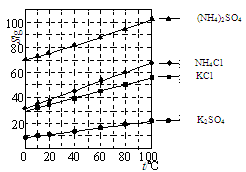
£Ø2£©¼ģŃéK2SO4ѳʷŹĒ·ńŗ¬ÓŠĀČ»ÆĪļŌÓÖŹµÄŹµŃé²Ł×÷ŹĒ£ŗ ”£
£Ø3£©·“Ó¦IVµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©Mn3O4ÓėÅØŃĪĖį¼ÓČČŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø5£©ĻĀĶ¼ŹĒģŃÉÕMnSO4?H2OŹ±ĪĀ¶ČÓėŹ£Óą¹ĢĢåÖŹĮæ±ä»ÆĒśĻß”£
¢ŁøĆĒśĻßÖŠB¶ĪĖł±ķŹ¾ĪļÖŹµÄ»ÆѧŹ½ĪŖ ”£
¢ŚģŃÉÕ¹ż³ĢÖŠ¹ĢĢåĆĢŗ¬ĮæĖęĪĀ¶ČµÄÉżø߶ųŌö“󣬵«µ±ĪĀ¶Č³¬¹ż1000”ꏱ£¬ŌŁĄäČ“ŗ󣬲āµĆ²śĪļµÄ×ÜĆĢŗ¬Įæ·“¶ų¼õŠ””£ŹŌ·ÖĪö²śĪļ×ÜĆĢŗ¬Įæ¼õŠ”µÄŌŅņ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĪļÖŹµÄ¹¤ŅµÖʱøŌĄķµÄ·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ
A£®ŅŅ“¼£ŗC6H12O6 2CH3CH2OH+2CO2”ü 2CH3CH2OH+2CO2”ü |
B£®ŅŅĻ©£ŗCH3CH2OH CH2=CH2”ü+ H2O CH2=CH2”ü+ H2O |
C£®ŃĪĖį£ŗNaCl+H2SO4 NaHSO4+HCl”ü NaHSO4+HCl”ü |
| D£®“æ¼ī£ŗ2NaCl+2NH3+CO2+H2O ”ś Na2CO3”ż+ 2NH4Cl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ¹ŲÓŚ¹¤ŅµÉś²śĖµ·Ø“ķĪóµÄŹĒ( )
| A£®ŌŚŗīŹĻÖĘ¼ī¹¤ŅµÖŠ£¬Ļņ±„ŗĶĀČ»ÆÄĘČÜŅŗÖŠĻČĶØČė°±Ęų£¬ŗóĶØČė¶žŃõ»ÆĢ¼ |
| B£®ŌŚĮņĖį¹¤Ņµ”¢ŗĻ³É°±¹¤Ņµ”¢ĻõĖį¹¤ŅµÖŠ£¬½Ō²ÉÓĆŃ»·²Ł×÷ĢįøßŌĮĻĄūÓĆĀŹ |
| C£®ŌŚĀČ¼ī¹¤Ņµ£¬µē½ā²Ū±»Ąė×Ó½»»»Ä¤øō³ÉŅõ¼«ŹŅŗĶŃō¼«ŹŅ |
| D£®¹¤ŅµÉĻ²ÉÓƵē½āČŪČŚĀČ»ÆĀĮµÄ·½·ØÖĘČ”½šŹōĀĮ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŗĻ³ÉNH3ĖłŠčµÄH2æÉÓÉĆŗÓėH2O·“Ó¦ÖĘµĆ£¬ĘäÖŠÓŠŅ»²½·“Ó¦ĪŖCO(g)£«H2O(g) CO2(g)£«H2(g)””¦¤H<0”£ÓūĢįøßCO×Ŗ»ÆĀŹæɲÉÓƵķ½·ØæÉÄÜÓŠ£ŗ¢Ł½µµĶĪĀ¶Č£»¢ŚŌö“óŃ¹Ēæ””¢ŪŹ¹ÓĆ“ß»Æ¼Į””¢ÜŌö“óCOµÄÅØ¶Č£»¢ŻŌö“óĖ®ÕōĘųµÄÅØ¶Č£¬ĘäÖŠÕżČ·µÄ×éŗĻŹĒ(””””)”£
CO2(g)£«H2(g)””¦¤H<0”£ÓūĢįøßCO×Ŗ»ÆĀŹæɲÉÓƵķ½·ØæÉÄÜÓŠ£ŗ¢Ł½µµĶĪĀ¶Č£»¢ŚŌö“óŃ¹Ēæ””¢ŪŹ¹ÓĆ“ß»Æ¼Į””¢ÜŌö“óCOµÄÅØ¶Č£»¢ŻŌö“óĖ®ÕōĘųµÄÅØ¶Č£¬ĘäÖŠÕżČ·µÄ×éŗĻŹĒ(””””)”£
| A£®¢Ł¢Ś¢Ū | B£®¢Ü¢Ż | C£®¢Ł¢Ż | D£®¢Ż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com