
 ��ȡ�ķ����� ��
��ȡ�ķ����� ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʧȥ���ӵ����� | B��ԭ�Ӻ�����11�����ӵ�Ԫ�� |
| C�������ᷴӦ������ | D��ԭ�ӵ������ֻ��һ�����ӵ�Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��X�� | B��X2�� | C��X3�� | D��X4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������غ�ԭ�� |
| B�������ܼ�ͼ�е�˳�� 3d ��������� 4s ����� |
| C�����ع��� |
| D������������ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| R | Ԫ��������������������������ԭ����������� |
| T | �����������Ǵ�����������2�� |
| X | Ԫ���������+7�� |
| Y | �������ڽ���Ԫ����ԭ�Ӱ뾶��С |
| Z | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
 ��Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������
��Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������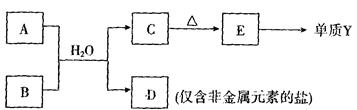
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CA3��g��
2CA3��g�� .0 L��
.0 L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
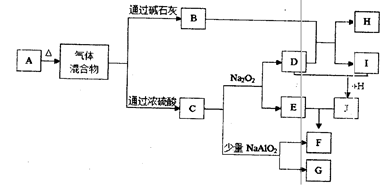
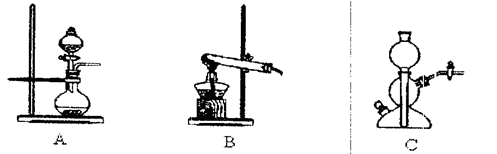
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com