
ij�������� FeCl2���ʵ� FeCl3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ�鲽�����£�
�� ȷ����mg ��Ʒ��
�� ����Ʒ�м��� 10 mL 5 mol · Lһ1�����ᣬ�ټ�������ˮ�����Ƴ� 250 mL ��Һ�� �� ��ȡ 25 mL ���� �� ����õ���Һ������ 3 mL ��ˮ������ʹ֮��ȫ��Ӧ��
�� ����Ѹ�ټ�����������Ϊ 10 ���İ�ˮ����������ֽ��裬ʹ֮��ȫ������
�� ���ˣ�������ϴ�Ӻ����ա���ȴ�����������ء�
���������������ش�
��1���ܽ���ƷʱҪ�������ᣬ��Ŀ����_______________________________________
��2������ 250 mL ��Һʱ�����貣�������ձ��⣬�����õ��IJ���������_________________________________________________
��3��������ˮʱ������Ӧ�����ӷ���ʽ�ǣ�
____________________________________________________
��4������������Ϊ W1 g �����������պ������������� W2 g ������Ʒ����Ԫ�ص�����������_____________________________
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵��������� �� ��
A��ʳ����ڱ����л����ʳ����ʵ�����
B���صĽ�����ǿ�����Լ���ˮ�ķ�Ӧ���Ƶķ�Ӧ����
C��2 mol SO2��1 mol O2��Ϻ��ַ�Ӧ����2 mol SO3
D����۳��Ͻ��̻��ԭ������۷۳��������������Ӵ�������������ը
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�л���A��B��C��D��E��F������ת����ϵ��A�����ϩ����E�Dz�����ˮ�Ҿ�����ζ����ɫҺ�壬��Է���������C��2����FΪ�߷��ӻ���������ͼ��ϵ�ش����⣺
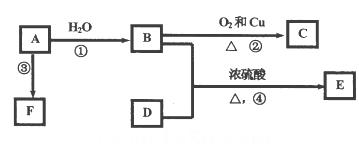
(1)д��C�Ľṹ��ʽ��__________��
(2)д��B��D�й����ŵ����ƣ�__________��D__________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��_______________________________��
��______________________________��
(4)����B����ֱ�ӱ�����ΪD����Ҫ������Լ���__________��ʵ���������________________________________________ ��
(5)����ʯ�ͻ�������Ҫ��Ʒ����Ũ����������£�����50�桫60����Ũ���ᷢ����Ӧ��д����Ӧ�Ļ�ѧ����ʽ____________________���÷�Ӧ����____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Լ���һ���Լ���BaCl2��NaOH��KI�ͱ�����ƿ��ɫˮ��Һ���ǣ� ��
A.NH4Cl B��Na2SO4 C�� FeCl3 D��ϡH2S04
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��Ԫ�����ڱ������ڵ�һ���֣�A��B����Ԫ�ؿ����γɹ��ۻ�����B2A2������������ԭ�ӵ��������ﵽ8�����ӵ��ȶ��ṹ������˵������ȷ���ǣ� ��
A���û�����������״̬���ܵ���
B���û�����Ľṹʽ��Cl-S-S-Cl
C�û������γɵľ����Ƿ��Ӿ���
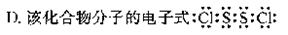
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���һ�������¼��� ��ӳɷ�Ӧ��Ҳ����ȡ����Ӧ��������ʹKMnO4������Һ��ɫ���ǣ� ��
A������ B���� C����ϩ D���Ҵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ3X(g)+Y(g)  ��2Z(g)+2W(g)��2 L�ܱ������н��У�5m in��Y������0.5
��2Z(g)+2W(g)��2 L�ܱ������н��У�5m in��Y������0.5
mol����˷�Ӧ��ƽ������vΪ�� ��
A��v (X)=0.05mol��L-1��min-1 B��v (Y)= 0.10mol��L-1��min-1
C��v (Z)=0.10mol��L-1��min-1 D��v (W)=0.05mol��L-1��s-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ӽṹʾ��ͼ
���ӵĺ˵��������Z����Ȧ���𡱣�Ȼ��ֲ�д�����������磺
Na________��Na��________��S________��S2��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ��һ�ֽ��壬���ҵ��������ⵥ�ʣ����Գ������Ե���ɫ�������ֽ����ۺ�ϡNa2SO4��Һ��ϣ�װ�ڰ�Ĥ�У�������ʢ������ˮ���ձ��ڣ���һ��ʱ���ȡ�ձ���Һ�����ʵ�飬��֤����Ĥ���������� ( ��
A������BaCl2��Һ������ɫ���� B�������ˮ������
C������BaCl2��Һ�ް�ɫ�������� D�������ˮ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com