
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棻���������۵�Ϊ231�档���������������������װһ��ʵ��װ�á������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ������˷�Ӧ���̷ų��������ȣ���
��ش����и������⡣
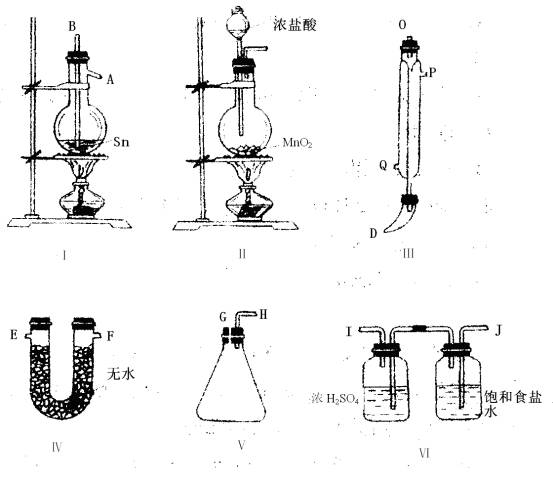
��1���ò����ܣ�δ��������������װ�ã���ȷ��˳���ǣ�����ӿڵĴ�����ĸ���� ���ӣ� ������ ���ӣ� ������ ���ӣ� ������ ���ӣ� ������ ���ӣ� ����
��2��װ�â������������� ��������������װ�â����������������� ���� ��
��3������������ȴˮ�������Ǵ����� ���� ���룬������ ���� ������
��4��ʵ��ʱӦ�ȵ�ȼ ���ƾ��ƣ������¶�Ӧ�������� �棬������ ����ֹͣ���ȡ�
��5����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ�������������������������������� ��
��6���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ��������������������������� ��
��7��Ϊ�˷�ֹ��Ⱦ��������װ�õ����Ӧ������������ ���������������� ��
��1����B���ӣ�J������I���ӣ�K������A���ӣ�C������D���ӣ�G������H���ӣ�E������F�� ��2����ȥ�����е�HCl��ˮ������ֹ�����е�ˮ���������ռ�SnCl4�ļ���ƿ�� ��3��Q�� P ��4��I ��231�������ۻ� ��5��SnCl4��2H2O��SnO2��4HCl�� ��6���а�ɫ�������� ��7����ʢ��NaOH������Һ���ձ����ն��������
|
����Ҫ��ɵķ�Ӧ�ǣ�Sn(��)��2Cl2(��)��SnCl4Ϊ�ˣ�Ӧ����ʹ����1��Sn�ۻ�����2���Ʊ����﴿����Cl2�������Ҹ�����Ŀ����ʾ��Ҫ��ֹSnCl4ˮ��ͼ���������Ӧ�����ڷų�������Ӧֹͣ�������ȡ� ��Ŀ������6��װ�ã�����Ϊ����Ӧװ�ã�������װ�ã�������װ�ã���������װ�ã���������װ�ã���������װ�á��ɴ˿��Իش� ��1�����Ӵ�����������������Ӧ�����������ܡ����������������������������������ҳ���ȷ�ӿڡ� ��2�����Ǿ���Cl2��װ�ã���ȻӦ���Ⱦ�������NaCl��Һ����ŨH2SO4������ӷ��ˣ�������ˮ����ʹ��Ӧ���ʽ��ͻ�ʧ�ܡ� ��3��������б�ã������е���ȴˮ�������¶�Q���룬�϶�P�������������ȴЧ�ʡ� ��4��������ӦӦ����ʹ�������ۻ�����Ŀ�Ѹ���Sn���۵㣬�����¶ȵ�Ȼ������ڸ��¶ȡ����ڷ�Ӧ���ڸ�������ƿ�н��У����Ҹ�Cl2��Ӧ������ų��������������Ե�����Sn�ۻ���Ӧֹͣ���ȡ� ��5�����Ȼ�����ˮ�ⷴӦ��Ӧ����ȡ����Ӧ��SnCl4��2H2O��SnO2��4HCl�� ��6��SnCl4��¶�ڿ����У�������ˮ��������SnO2��ɫС���壬�γɰ��̣�ͬʱ�ų�HCl���壬�γɰ��������а�ɫ���������� ��7����Ӧ�У������ж����Cl2���ݳ�����ˣ���ý���һ��ʢ�м�Һ������β����װ�ã���ӦΪ��Cl2��2NaOH��NaClO��NaCl��H2O
|


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)�ò�����(δ����)��������װ�ã���ȷ��˳����(����ӿڵĴ�����ĸ)( )��( )��( )��( )��( )��( )��( )��( )��( )��( )��?
(2)װ�â���������__________________��װ�â���������________________________��
(3)����������ȴˮ�������Ǵ�_____________���룬��_____________������
(4)ʵ��ʱӦ�ȵ�ȼ_____________���ƾ��ƣ������¶�Ӧ����_____________�棬��_____________����ֹͣ���ȡ�
(5)��֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________��
(6)�������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������____________________��
(7)Ϊ�˷�ֹ��Ⱦ��������װ�õ����Ӧ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棻���������۵�Ϊ231�棬�������������������װһ��ʵ��װ�ã������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ���(�˷�Ӧ���̷ų���������)��ش����и������⡣

A���ò�����(δ����)��������װ�ã���ȷ��˳����(����ӿڵĴ���ĸ)
( )��( )��( )��( )��( )��( )��( )��( )��( )��( )��
B��װ�â�������______��װ�â���������______��
C������������ˮ�������Ǵ�______���룬��________������
D��ʵ��ʱӦ�ȵ�ȼ_______���ƾ��ƣ������¶�Ӧ����______�棬��_______����ֹͣ���ȣ�
E����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ_______��
F���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������________��
G��Ϊ�˷�ֹ��Ⱦ��������װ�õ����Ӧ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������к�ƽ�������ڶ��������������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棬���������۵�Ϊ231�档װ��A�з�Ũ����B�з�MnO2�����������������������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ������˷�Ӧ���̷ų��������ȣ�����ش����и����⡣

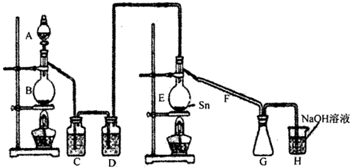
��1������ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��H�з�Ӧ�����ӷ���ʽ��_________________________________________________��
E�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��3��C��D�е��Լ��ֱ���_______________��____________________��
��4������A��B�����Ʒֱ���_____________��____________��F��������_____________��
��5��ʵ��ʱӦ�ȵ�ȼ_________���ƾ��ƣ������¶�Ӧ����________ �棬��________������ֹͣ���ȡ�
��6����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________________________________________________��
��7���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ƽ����ģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com