
¢ÅÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗpH”£ŹµŃ鏱£¬ĖūĻČÓĆÕōĮóĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»øÉŌļµÄ²£Į§°ōÕŗČ”ŹŌŃł½ųŠŠ¼ģ²ā”£
¢ŁÕāÖÖ“ķĪó²Ł×÷ £ØĢī”°Ņ»¶Ø”±/”°Ņ»¶Ø²»”±/”°²»Ņ»¶Ø”±£©»įµ¼ÖĀŹµŃé½į¹ūÓŠĪó²ī”£
¢ŚČō°““Ė·Ø·Ö±š²ā¶Øc(H+)ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ ”£
¢Ę”¢ÓĆŅŃÖŖÅØ¶ČµÄ NaOH ČÜŅŗ²ā¶Øij HClČÜŅŗµÄÅØ¶Č£¬²Īæ¼ÓŅĶ¼£¬“Ó±ķÖŠŃ”³öÕżČ·ŠņŗÅ _____________
|
ŠņŗÅ |
׶ŠĪĘæÖŠČÜŅŗ |
µĪ¶Ø¹ÜÖŠČÜŅŗ |
Ń”ÓĆÖøŹ¾¼Į |
|
|
A |
¼ī |
Ėį |
ŹÆŠ¾ |
£ØŅŅ£© |
|
B |
Ėį |
¼ī |
·ÓĢŖ |
£Ø¼×£© |
|
C |
¼ī |
Ėį |
¼×»ł³Č |
£Ø¼×£© |
|
D |
Ėį |
¼ī |
·ÓĢŖ |
£ØŅŅ£© |
¢Ē”¢ÓƱź×¼µÄNaOHµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬Ń”ÓĆ·ÓĢŖĪŖÖøŹ¾¼Į£¬Ōģ³É²ā¶Ø½į¹ūĘ«øßµÄŌŅņæÉÄÜŹĒ ”£
A. ÅäÖʱź×¼ČÜŅŗµÄĒāŃõ»ÆÄĘÖŠ»ģÓŠNa2CO3ŌÓÖŹ
B. µĪ¶ØÖÕµć¶ĮŹżŹ±£¬ø©ŹÓµĪ¶Ø¹ÜµÄæĢ¶Č£¬ĘäĖü²Ł×÷¾łÕżČ·
C. Ź¢×°Ī“ÖŖŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¹ż£¬Ī“ÓĆ“ż²āŅŗČóĻ“
D. µĪ¶Øµ½ÖÕµć¶ĮŹżŹ±·¢ĻÖµĪ¶Ø¹Ü¼ā×ģ“¦Šü¹ŅŅ»µĪČÜŅŗ
E. Ī“ÓƱź×¼ŅŗČóĻ“¼īŹ½µĪ¶Ø¹Ü
¢Č”¢ŅŃÖŖµĪ¶Ø¹Ü֊װӊÅضČĪŖ0.112mol/LµÄŃĪĖį”£ÖšµĪ¼ÓČėµ½×°ÓŠĒāŃõ»ÆÄʵÄČÜŅŗµÄ׶ŠĪĘæÖŠ”£æŖŹ¼Ź±¶ĮŹż¼°Ē”ŗĆ·“Ó¦Ź±ŃĪĖįµÄ¶ĮŹż¼ūĻĀ±ķ”£
|
ŹµŃé ±ąŗÅ |
“ż²āĒāŃõ»ÆÄĘČÜŅŗĢå»ż£ØmL£© |
µĪ¶ØæŖŹ¼¶ĮŹż£ØmL£© |
µĪ¶Ø½įŹų¶ĮŹż£ØmL£© |
ĻūŗÄŃĪĖįĢå»ż£ØmL£© |
|
¢Ł |
25£®00 |
0£®02 |
26£®40 |
|
|
¢Ś |
25£®00 |
0£®04 |
25£®81 |
|
|
¢Ū |
25£®00 |
0£®03 |
25£®78 |
|
|
¢Ü |
25£®00 |
0£®20 |
25£®96 |
|
ŹŌ¼ĘĖć“ż²āµÄĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæÅضČ= .
¢Å¢Ł²»Ņ»¶Ø ¢ŚŃĪĖį ¢Ę D ¢Ē ADE ¢Č 0.1158mol/L
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©¢ŁÓĆÓĆÕōĮóĖ®ČóŹŖŗópHŹŌÖ½²āĮæČÜŅŗµÄpHŹ±£¬Ļąµ±ÓŚ°ŃŌČÜŅŗĻ”ŹĶ£¬ĖłŅŌ²»Ņ»¶Ø²śÉśĪó²ī”£ĄżČēŌČÜŅŗĻŌÖŠŠŌ£¬ŌņŹĒ²»Ó°ĻģµÄ”£
¢ŚÓÉÓŚ“×ĖįŹĒČõĖį£¬“ęŌŚµēĄėĘ½ŗā£¬¶ųĻ”ŹĶ“Ł½ųµēĄė£¬ĖłŅŌČō°““Ė·Ø·Ö±š²ā¶Øc(H+)ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄÓ¦øĆŹĒŃĪĖį”£
£Ø2£©ÓĆŅŃÖŖÅØ¶ČµÄ NaOH ČÜŅŗ²ā¶Øij HClČÜŅŗµÄÅØ¶Č£¬ŌņŠčŅŖ¼īŹ½µĪ¶Ø¹Ü£¬¼“Ń”ŌńŅŅ£»ÓÖŅņĪŖŅ»°ć²»Ń”ŌńŹÆČļŹŌŅŗ×÷ÖøŹ¾¼Į£¬ĖłŅŌÓ¦øĆŃ”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£¬¼““š°øŃ”D”£
£Ø3£©ÓÉÓŚµČÖŹĮæµÄĢ¼ĖįÄĘĻūŗĵÄŃĪĖįŠ”ÓŚµČÖŹĮæµÄĒāŃõ»ÆÄĘĻūŗĵÄŃĪĖį£¬ĖłŅŌŌŚµĪ¶Ø¹ż³ĢÖŠĻūŗĵļīŅŗĢå»żĘ«¶ą£¬Ōņ²ā¶Ø½į¹ūĘ«øߣ»µĪ¶Ø¹ÜµÄæĢ¶Č×ŌÉĻ¶ųĻĀŹĒÖš½„Ōö“óµÄ£¬ĖłŅŌµĪ¶ØÖÕµć¶ĮŹżŹ±£¬ø©ŹÓµĪ¶Ø¹ÜµÄæĢ¶Č£¬Ōņ¶ĮŹżĘ«Š”£¬ĖłŅŌ²ā¶Ø½į¹ūĘ«µĶ£»CÖŠŹĒ±ŲŅŖÓ°ĻģµÄ£»µĪ¶Øµ½ÖÕµć¶ĮŹżŹ±·¢ĻÖµĪ¶Ø¹Ü¼ā×ģ“¦Šü¹ŅŅ»µĪČÜŅŗ£¬ŌņĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żĘ«¶ą£¬ĖłŅŌ²ā¶Ø½į¹ūĘ«øߣ»Ī“ÓƱź×¼ŅŗČóĻ“¼īŹ½µĪ¶Ø¹Ü£¬ŌņĻąµ±ÓŚĻ”ŹĶĒāŃõ»ÆÄĘČÜŅŗ£¬ĖłŅŌĻūŗÄĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żĘ«¶ą£¬²ā¶Ø½į¹ūĘ«øߣ¬Ņņ“Ė“š°øŃ”ADE”£
£Ø4£©øł¾Ż±ķÖŠŹż¾ŻæÉÖŖ£¬ĻūŗĵÄŃĪĖįĢå»ż·Ö±šŹĒ25.98ml”¢25.77ml”¢25.75ml”¢25.76ml£¬ĖłŅŌĻūŗÄŃĪĖįµÄĘ½¾łÖµŹĒ£Ø25.98ml£«25.77ml£«25.75ml£«25.76ml£©”Ā4£½25.87ml£¬ĖłŅŌĒāŃõ»ÆÄĘČÜŅŗµÄÅØ¶ČŹĒ25.87ml”Į0.112mol/L”Ā25.00ml£½0.1158mol/L”£
æ¼µć£ŗæ¼²épHŹŌÖ½µÄŹ¹ÓĆ”¢ÖŠŗĶµĪ¶ØµÄÖŠŅĒĘ÷ŗĶÖøŹ¾¼ĮµÄŃ”Ōń£¬Īó²ī·ÖĪöµČ
µćĘĄ£ŗŌŚ½ųŠŠÖŠŗĶµĪ¶ØĪó²ī·ÖĪöŹ±£¬ŠčŅŖŅĄ¾Ż £¬¼“ŌŚŹµŃé¹ż³ĢÖŠ£¬V1ŗĶC2ŹĒ²»±äµÄ£¬Ņņ“Ė¹Ų¼üŹĒ·ÖĪö²Ł×÷ŹĒČēŗĪµ¼ÖĀV2±ä»ÆµÄ¼“æÉ”£
£¬¼“ŌŚŹµŃé¹ż³ĢÖŠ£¬V1ŗĶC2ŹĒ²»±äµÄ£¬Ņņ“Ė¹Ų¼üŹĒ·ÖĪö²Ł×÷ŹĒČēŗĪµ¼ÖĀV2±ä»ÆµÄ¼“æÉ”£


 Ļ°Ģā¾«Ń”ĻµĮŠ“š°ø
Ļ°Ģā¾«Ń”ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“µĪ¶Ø²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£®ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“µĪ¶Ø²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬Ń”Ōń·ÓĢŖ×÷ÖøŹ¾¼Į£®ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ| µĪ¶Ø“ĪŹż | “ż²āĒāŃõ»ÆÄĘ ČÜŅŗµÄĢå»ż/mL |
0.1000mol/LŃĪĖįµÄĢå»ż£ØmL£© | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ČÜŅŗĢå/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
| µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ŁøĆѧɜµÄ²Ł×÷ŹĒ__________(Ģī”°ÕżČ·µÄ”±»ņ”°“ķĪóµÄ”±)£¬ĘäĄķÓÉŹĒ___________________”£
¢ŚČē²»ÕżČ·ĒėĖµĆ÷ĄķÓÉ£¬Ķ¬Ź±Ēė·ÖĪöŹĒ·ńŅ»¶ØÓŠĪó²ī£æ“š_____________________”£
¢ŪČōÓĆ“Ė·Ø·Ö±š²ā¶Ø£ŪH+£ŻĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ_________________£¬ŌŅņŹĒ_____________________________________________”£
(2)ČōŅŌĢś°ō”¢Ģ¼°ō”¢µ¼ĻßŗĶĀČ»ÆĢśČÜŅŗĪŖÓĆĘ·Éč¼ĘŌµē³Ų”£
øŗ¼«²ÄĮĻĪŖ_______________£¬µē¼«·“Ó¦Ź½ĪŖ_______________£»
Õż¼«²ÄĮĻĪŖ_______________£¬µē¼«·“Ó¦Ź½ĪŖ_______________£»
µē³Ų×Ü·“Ó¦Ź½ĪŖ______________________________________________________”£
(3)»š¼żĶĘ½ųĘ÷ÖŠŹ¢ÓŠĒ滹Ō¼ĮŅŗĢ¬ėĀ(N2H4)ŗĶĒæŃõ»Æ¼ĮŅŗĢ¬Ė«ŃõĖ®”£µ±ĖüĆĒ»ģŗĻ·“Ó¦Ź±£¬¼“²śÉś“óĮæµŖĘųŗĶĖ®ÕōĘų£¬²¢·Å³ö“óĮæČČ”£ŅŃÖŖ£ŗ0.4 molŅŗĢ¬ėĀÓė×ćĮæµÄŅŗĢ¬Ė«ŃõĖ®·“Ó¦£¬Éś³ÉµŖĘųŗĶĖ®ÕōĘų£¬·Å³ö256.652 kJµÄČČĮ攣
¢Ł·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ__________________”£
¢ŚÓÖŅŃÖŖH2O(l)![]() H2O(g) ¦¤H=+44 kJ”¤mol-1£¬Ōņ
H2O(g) ¦¤H=+44 kJ”¤mol-1£¬Ōņ
¢Ū“Ė·“Ó¦ÓĆÓŚ»š¼żĶĘ½ų£¬³żŹĶ·Å“óĮæČČŗĶæģĖŁ²śÉś“óĮæĘųĢåĶā»¹ÓŠŅ»øöŗÜ“óµÄÓŵćŹĒ_________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ń”¢£Ø8·Ö£©ÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗµÄpH”£ŹµŃ鏱£¬ĖūĻČÓĆÕōĮóĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»øÉŌļµÄ²£Į§°ōõ“Č”ŹŌŃł½ųŠŠ¼ģ²ā”£
£Ø1£©øĆѧɜµÄ²Ł×÷ŹĒ”””””” ””””£ØĢī”°ÕżČ·µÄ”±»ņ”°“ķĪóµÄ”±£©”£
£Ø2£©Čē²»ÕżČ·£¬ŹĒ·ńŅ»¶ØÓŠĪó²ī£æ“š£ŗ””””””””””£ØĢī”°ŹĒ”±»ņ”°·ń”±£©
£Ø3£©Čō°““Ė·Ø·Ö±š²ā¶ØC£ØH£«£©ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ””””””””£¬ŌŅņŹĒ”””””””” ”””””””””£
¢ņ”¢£Ø10·Ö£©
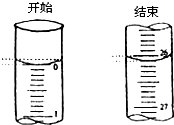
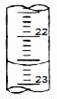
£Ø1£©ČēĶ¼±ķŹ¾50mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬Ęä¶ĮŹżŹĒ mL
£Ø2£©ŅŅ¶žĖįĖ×Ćū²ŻĖį£¬Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ÓūĢ½¾æ²ā¶Ø
|
¢Ł”¢ĻĀĮŠŅĒĘ÷ŌŚ½ųŠŠµĪ¶ØŹ±£¬¾ų²»æÉŅŌŹĀĻČČóĻ“µÄŹĒ £ØĢī±ąŗÅ£©”£
¼×£®ĖįŹ½µĪ¶Ø¹Ü ŅŅ£®¼īŹ½µĪ¶Ø¹Ü ±ū£®25mLĮæĶ² ¶”£®×¶ŠĪĘæ
¢Ś”¢µĪ¶ØŹ±£¬½«KMnO4±ź×¼Ņŗ×°ŌŚ µĪ¶Ø¹ÜÖŠ”£
¢Ū”¢ ±¾ŹµŃéµĪ¶Ø“ļµ½ÖÕµćµÄ±źÖ¾æÉŅŌŹĒ
ӣ
¢Ü”¢ ČōµĪ¶ØÖÕµćŹ±ø©ŹÓµĪ¶Ø¹ÜæĢ¶Č£¬ŌņÓÉ“Ė²āµĆµÄxÖµ»į £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗÓ±±Ź”»Ęęč֊ѧø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ÓƱź×¼NaOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬ÓĆ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ĻĀĮŠ²Ł×÷ÖŠ»įµ¼ÖĀŹµŃé½į¹ūĘ«µĶµÄŹĒ ”£
¢Ł¼īŹ½µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊÓƱź×¼ŅŗČóĻ“
¢ŚÓĆĖįŹ½µĪ¶Ø¹Ü¼Ó“ż²āŅŗŹ±£¬øÕÓĆÕōĮóĖ®Ļ“¾»ŗóµÄµĪ¶Ø¹ÜĪ“ÓĆ“ż²āŅŗČóĻ“
¢Ū׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊÓĆ“ż²āŅŗČóĻ“
¢ÜµĪ¶ØĒ°µĪ¶Ø¹Ü¼ā¶ĖÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§
¢ŻÖÕµć¶ĮŹżŹ±ø©ŹÓ£¬ĘäĖū¶ĮŹż·½·ØÕżČ·
£Ø2£©ÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗµÄpH”£ŹµŃ鏱ĖūĻČÓĆÕōĮóĖ®ŹŖČópHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»µÄ²£Į§°ōÕŗČ”ŹŌŃł½ųŠŠ¼ģ²ā”£
¢ŁøĆѧɜµÄ²Ł×÷ £ØĢī”°ÕżČ·”±»ņ”°²»ÕżČ·”±£©£¬Čē²»ÕżČ·£¬Ēė·ÖĪöŹĒ·ńŅ»¶ØÓŠĪó²ī ²¢ĖµĆ÷ĄķÓÉ£ØČōÕżČ·Ōņ“ĖĪŹ²»ÓĆ×÷“š£©
²¢ĖµĆ÷ĄķÓÉ£ØČōÕżČ·Ōņ“ĖĪŹ²»ÓĆ×÷“š£©
ӣ
¢ŚČō°““Ė·Ø·Ö±š²ā¶Øc(H+)ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ £¬ŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģø£½ØŹ”ø߶žĻĀѧʌµŚŅ»Ń§¶Īæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
¢ń”¢£Ø8·Ö£©ÓŠŅ»Ń§ÉśŌŚŹµŃéŹŅ²āijČÜŅŗµÄpH”£ŹµŃ鏱£¬ĖūĻČÓĆÕōĮóĖ®ČóŹŖpHŹŌÖ½£¬Č»ŗóÓĆ½ą¾»øÉŌļµÄ²£Į§°ōõ“Č”ŹŌŃł½ųŠŠ¼ģ²ā”£
£Ø1£©øĆѧɜµÄ²Ł×÷ŹĒ”””””” ””””£ØĢī”°ÕżČ·µÄ”±»ņ”°“ķĪóµÄ”±£©”£
£Ø2£©Čē²»ÕżČ·£¬ŹĒ·ńŅ»¶ØÓŠĪó²ī£æ“š£ŗ””””””””””£ØĢī”°ŹĒ”±»ņ”°·ń”±£©
£Ø3£©Čō°““Ė·Ø·Ö±š²ā¶ØC£ØH£«£©ĻąµČµÄŃĪĖįŗĶ“×ĖįČÜŅŗµÄpH£¬Īó²ī½Ļ“óµÄŹĒ””””””””£¬ŌŅņŹĒ”””””””” ”””””””””£
¢ņ”¢£Ø10·Ö£©
£Ø1£©ČēĶ¼±ķŹ¾50mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬Ęä¶ĮŹżŹĒ mL
£Ø2£©ŅŅ¶žĖįĖ×Ćū²ŻĖį£¬Ä³»ÆѧѧĻ°Š”×éµÄĶ¬Ń§ÓūĢ½¾æ²ā¶Ø
|
¢Ł”¢ĻĀĮŠŅĒĘ÷ŌŚ½ųŠŠµĪ¶ØŹ±£¬¾ų²»æÉŅŌŹĀĻČČóĻ“µÄŹĒ £ØĢī±ąŗÅ£©”£
¼×£®ĖįŹ½µĪ¶Ø¹Ü ŅŅ£®¼īŹ½µĪ¶Ø¹Ü ±ū£®25 mLĮæĶ² ¶”£®×¶ŠĪĘæ
¢Ś”¢µĪ¶ØŹ±£¬½«KMnO4±ź×¼Ņŗ×°ŌŚ µĪ¶Ø¹ÜÖŠ”£
¢Ū”¢ ±¾ŹµŃéµĪ¶Ø“ļµ½ÖÕµćµÄ±źÖ¾æÉŅŌŹĒ
ӣ
¢Ü”¢ ČōµĪ¶ØÖÕµćŹ±ø©ŹÓµĪ¶Ø¹ÜæĢ¶Č£¬ŌņÓÉ“Ė²āµĆµÄxÖµ»į £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com