
�����仯���ﳣ��Ӧ����ұ�𡢻�������ơ���ҩ����֯����ҵ����ʹ�ú�ķ�ˮ�����к��۸��Ļ���������Ժ�ǿ����ҵ��������ȡ����ѭ�����շ�ֹ������Ⱦ��

���������գ�
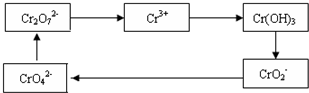
1����Cr2O72�����ӵķ�ˮ��������������Fe3O4��������Ӧ�����ӷ���ʽ���£�
________Cr2O72����________Fe3O4��________H+��________Cr3+��________Fe3+��________H2O
��ƽ�������ӷ�Ӧ����ʽ���������Ӧ�е���ת�Ƶķ������Ŀ��
2����������Ӧ�����Һ�м������Һ���÷�ˮ��pH����8��10�������ʵ����¶ȣ�ʹ�����еĽ���������ȫ��ת��Ϊ������д���Ӹó����з����Cr(OH)3�����ӷ���ʽ________��________��
3���������õ�Cr(OH)3�����������ֿ�ת���K2Cr2O7��������K2Cr2O7������ȷ�ⶨNa2S2O3��Һ�����ʵ���Ũ�ȣ��������£�
��Cr2O72����6I����14H+��3I2��2Cr3+��7H2O
��2S2O32����I2��S4O62����2I��
ȷ��ȡ������K2Cr2O70.1225 g�������Һ����Na2S2O3��Һ�ζ�������Na2S2O3��Һ25.00 mL����Na2S2O3��Һ�����ʵ���Ũ��Ϊ________(������λ��Ч����)��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ���������������һѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�����仯���ﳣ��Ӧ����ұ�𡢻�������ơ���ҩ����֯����ҵ����ʹ�ú�ķ�ˮ�����к��۸��Ļ���������Ժ�ǿ����ҵ��������ȡ����ѭ�����շ�ֹ������Ⱦ��
���������գ�
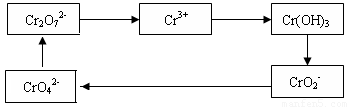
��1����Cr2O72-���ӵķ�ˮ��������������Fe3O4��������Ӧ�����ӷ���ʽ���£�
Cr2O72- + Fe3O4 + H+ �� Cr3+ + Fe3+ + H2O
��ƽ�������ӷ�Ӧ����ʽ���������Ӧ�е���ת�Ƶķ������Ŀ��
��2����������Ӧ�����Һ�м������Һ���÷�ˮ��pH����8��10�������ʵ����¶ȣ�ʹ�����еĽ���������ȫ��ת��Ϊ������д���Ӹó����з����Cr(OH)3�����ӷ���ʽ ��
��
��3���������õ�Cr(OH)3�����������ֿ�ת���K2Cr2O7��������K2Cr2O7������ȷ�ⶨNa2S2O3��Һ�����ʵ���Ũ�ȣ��������£�
�� Cr2O72- +6I- + 14H+ ��3I2 + 2Cr3+ + 7H2O �� 2S2O32- + I2��S4O62- + 2I-
ȷ��ȡ������K2Cr2O7 0.1225g �������Һ����Na2S2O3��Һ�ζ�������Na2S2O3��Һ25.00mL����Na2S2O3��Һ�����ʵ���Ũ��Ϊ ��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��������ɽ��������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�����仯�������ִ���ҵ�ϵ�Ӧ�ù㷺�������ڵ�ƣ�������ë��Ƥ���������������ϣ�����ӡˢ��������ý���ȡ����������ϸ��������彡���кܴ�Σ����
��ij�������Ƹ﹤ҵ������Cr��III�������������ù������£������ȡҺ�н���������Ҫ��Cr3+�������Fe3����Al3+��Ca2+��Mg2+����
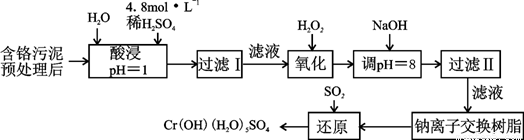
���������ӳ�����������������ʽ����ʱ��Һ��pH���±���
|
������ |
Fe3+ |
Fe2+ |
Mg2+ |
Al3+ |
Cu2+ |
Cr3+ |
|
��ʼ����ʱ��pH |
1.9 |
7.0 |
���� |
���� |
4.7 |
���� |
|
������ȫʱ��pH |
3.2 |
9.0 |
11.1 |
8 |
6.7 |
9 ����9�ܽ⣩ |
��1��ʵ������18.4 mol��L��1��Ũ��������250 mL 4.8 mol��L��1��������Һ�����õIJ����������ձ����������������ܣ�һ���ܾ�ȷ��ȡһ�����Һ����������⣬���� ��
��2�����ʱ��Ϊ����߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�� �����ٴ�һ�㣩��
��3��������Һ��pH=8��Ϊ�˳�ȥ ���ӡ������ӽ�����֬��ԭ��Ϊ��
Mn����nNaR��MRn��nNa�����������ĵ����������� ��
��4��Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s)  Cr3+(aq)+3OH��(aq)
Cr3+(aq)+3OH��(aq)
�����£�Cr(OH)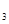 ���ܶȻ�Ksp= c(Cr3+)• c3(OH��)=10��32��Ҫʹc(Cr3+)����10��5mol/L����Һ��pHӦ����
��
���ܶȻ�Ksp= c(Cr3+)• c3(OH��)=10��32��Ҫʹc(Cr3+)����10��5mol/L����Һ��pHӦ����
��
��5����ԭ���̷������·�Ӧ������ƽ���� Na2Cr2O7�� SO2��
= Cr(OH) (H2O)5SO4�� Na2SO4��
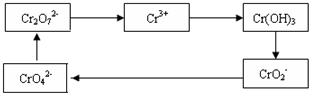
��ҵ��ˮ�г�����һ������Cr2O72����CrO42�������ǻ�����༰��̬ϵͳ�����ܴ���������д�����
����һ�ִ�������Ϊ��ⷨ���÷���Fe���缫����⺬Cr2O72�������Է�ˮ�����ŵ����У�����������ҺpH���ߣ�����Cr(OH)3��������Fe���缫��ԭ��Ϊ
������������ҺpH���ߵ�ԭ���ǣ��õ缫��Ӧ���ͣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com