
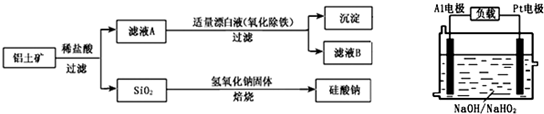
£Ø1£©µē¶ĘµÄŗ¬Ņå
µē¶ĘŹĒÓ¦ÓĆ___________ŌŚÄ³Š©½šŹō±ķĆę¶ĘÉĻŅ»±”²ćĘäĖū½šŹō»ņŗĻ½šµÄ¹ż³Ģ”£
£Ø2£©µē¶ĘµÄÄæµÄ
µē¶ĘµÄÄæµÄÖ÷ŅŖŹĒ___________________”£
£Ø3£©µē¶ĘµÄŌĄķ
Ńō¼«£ŗ_________________”£
Ņõ¼«£ŗ_________________”£
µē¶ĘŅŗ£ŗ______________________”£
£Ø4£©ĶµÄ¾«Į¶
¢Ł×°ÖĆŅŖĒó
Ńō¼«ŹĒ___________£¬Ņõ¼«ŹĒ___________£¬µē½āÖŹČÜŅŗŹĒ______________________”£
¢Ś»ÆѧŌĄķ
Ńō¼«·“Ó¦£ŗCu-2e-![]() Cu2+
Cu2+
Zn-2e-![]() Zn2+
Zn2+
Ņõ¼«·“Ó¦£ŗ__________________
¢Ūµē½āĢŲµć
a.“ÖĶÖŠµÄĶ”°ĒØŅĘ”±µ½“æĶÉĻ£»
b.CuSO4ČÜŅŗµÄÅضČ_________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”Äž²ØŹŠ°ĖŠ£ø߶žĻĀѧʌʌĩĮŖæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©øõ»Æѧ·įø»¶ą²Ź£¬ÓÉÓŚøõ¹āŌó¶ČŗĆ£¬³£½«øõ¶ĘŌŚĘäĖū½šŹō±ķĆę£¬Ķ¬Ģś”¢Äų×é³Éø÷ÖÖŠŌÄܵIJ»ŠāøÖ”£K2Cr2O7ŗĶCrO3“óĮæÓĆÓŚ÷·øļ”¢Ó”Č¾”¢ŃÕĮĻ”¢µē¶ĘµČ¹¤ŅµÖŠ£¬ŹĒ¹¤ŅµÉĻŌģ³ÉøõĪŪČ¾µÄÖ÷ŅŖŌŅņ”£½üĘŚ±©¹āµÄ”°¶¾½ŗÄŅ”±ŹĀ¼žÖŠ£¬¾ĶŹĒŅņĪŖÓĆ¹¤ŅµĘ¤øļµÄĻĀ½ÅĮĻ»ņĘĘʤŠ¬µČĪŖŌĮĻÖĘ³ÉµÄ¹¤ŅµĆ÷½ŗ±»Ć°³ä³ÉŹ³ÓĆĆ÷½ŗÖĘ³É½ŗÄŅ£¬Ōģ³É½ŗÄŅÄŚµÄøõŃĻÖŲ³¬±ź”£
£Ø1£©CrO3µÄČČĪČ¶ØŠŌ½Ļ²ī£¬¼ÓČČŹ±Öš²½·Ö½ā£¬Ęä¹ĢĢ岊ĮōĀŹĖęĪĀ¶ČµÄ±ä»ÆČēĶ¼ĖłŹ¾”£ 
¢ŁA µćŹ±Ź£Óą¹ĢĢåµÄ³É·ÖŹĒ £ØĢī»ÆѧŹ½£©”£
¢Ś“ÓæŖŹ¼¼ÓČȵ½ 750K Ź±×Ü·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø2£©Cr(¢ö)Ö÷ŅŖŅŌCrO42£ŗĶCr2O72£ŠĪĢ¬“ęŌŚ£¬ŌŚĖįŠŌĢõ¼žĻĀ¾ßÓŠŗÜĒæµÄŃõ»ÆŠŌ£¬ĖüĆĒŌŚČÜŅŗÖŠ“ęŌŚČēĻĀ×Ŗ»Æ£ŗCrO42£(»ĘÉ«)+2H+ Cr2O72£(³ČÉ«£©+H2O£»K=4.2”Į1014”£ČōŅŖŹ¹ČÜŅŗÓÉ»ĘÉ«±ä³ČÉ«£¬ŌņÓ¦²ÉČ”µÄ“ėŹ©ŹĒ ”£
Cr2O72£(³ČÉ«£©+H2O£»K=4.2”Į1014”£ČōŅŖŹ¹ČÜŅŗÓÉ»ĘÉ«±ä³ČÉ«£¬ŌņÓ¦²ÉČ”µÄ“ėŹ©ŹĒ ”£
| A£®¼ÓNaOH | B£®¼ÓŃĪĖį | C£®¼ÓĮņĖį | D£®¼ÓAgNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗž±±Ź”ĻåŃōŹŠµČĖÄŠ£øßČżÉĻѧʌʌ֊ĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
µē¶Ę³§Ōų²ÉÓĆÓŠĒčµē¶Ę¹¤ŅÕ£¬ÓÉÓŚÅŷŵķĻĖ®ÖŠŗ¬ÓŠµÄ¾ē¶¾CN-Ąė×Ó£¬¶ųÖš½„±»ĪŽĒčµē¶Ę¹¤ŅÕĢę“ś”£“¦ĄķÓŠĒčµē¶ĘµÄ·ĻĖ®Ź±£¬æÉŌŚ“߻ƼĮTiO2×÷ÓĆĻĀ£¬ĻČÓĆNaClO½«CN-Ąė×ÓŃõ»Æ³ÉOCN-£¬ŌŁŌŚĖįŠŌĢõ¼žĻĀ¼ĢŠų±»NaClOŃõ»Æ³ÉN2ŗĶCO2”£»·±£¹¤×÷ČĖŌ±ŌŚĆܱÕĻµĶ³ÖŠÓĆĻĀĶ¼×°ÖĆ½ųŠŠŹµŃ飬ŅŌÖ¤Ć÷“¦Ąķ·½·ØµÄÓŠŠ§ŠŌ£¬²¢²ā¶ØCN-±»“¦ĄķµÄ°Ł·ÖĀŹ”£½«ÅØĖõŗóŗ¬CN-Ąė×ÓµÄĪŪĖ®Óė¹żĮæNaClOČÜŅŗµÄ»ģŗĻŅŗ¹²200mL£ØĘäÖŠCN-µÄÅضČĪŖ0.05mol”¤L-1£©µ¹Čė¼×ÖŠ£¬ČūÉĻĻšĘ¤Čū£¬Ņ»¶ĪŹ±¼äŗ󣬓ņæŖĻšĘ¤ČūŗĶ»īČū£¬Ź¹ČÜŅŗČ«²æ·ÅČėŅŅÖŠ£¬¹Ų±Õ»īČū”£»Ų“šĻĀĮŠĪŹĢā£ŗ
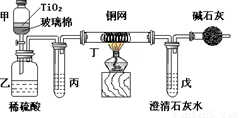
£Ø1£©ŅŅÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©ŅŅÖŠÉś³ÉµÄĘųĢå³żN2ŗĶCO2Ķā£¬»¹ÓŠø±²śĪļHCl¼°Cl2µČ£¬ÉĻŹöŹµŃéŹĒĶعż²ā¶Ø¶žŃõ»ÆĢ¼µÄĮæĄ“Č·¶Ø¶ŌCN-µÄ“¦ĄķŠ§¹ū”£Ōņ±ūÖŠ¼ÓČėµÄ³żŌÓŹŌ¼ĮŹĒ___________£ØĢī×ÖÄø£©
A£®±„ŗĶŹ³ŃĪĖ® B£®±„ŗĶNaHCO3ČÜŅŗ C£®ÅØNaOHČÜŅŗ D£®ÅØĮņĖį
£Ø3£©¶”ŌŚŹµŃéÖŠµÄ×÷ÓĆŹĒ ”£
×°ÓŠ¼īŹÆ»ŅµÄøÉŌļ¹ÜµÄ×÷ÓĆŹĒ ”£
£Ø4£©ĪģÖŠŹ¢ÓŠŗ¬Ca(OH)2 0.02molµÄŹÆ»ŅĖ®£¬ČōŹµŃéÖŠĪģÖŠ¹²Éś³É0.82 g³Įµķ£¬ŌņøĆŹµŃéÖŠ²āµĆCN-±»“¦ĄķµÄ°Ł·ÖĀŹµČÓŚ ”£ČōøĆ²āµĆÖµÓėŹµ¼Ź“¦ĄķµÄ°Ł·ÖĀŹĻą±ČĘ«µĶ£¬Ēė¼ņŅŖĖµĆ÷æÉÄܵÄČĪŅāŅ»øöŌŅņ ”£
£Ø5£©ĒėĢį³öŅ»øöÄÜĢįøß×¼Č·¶ČµÄ½ØŅé£ØŅŖÓŠæɲŁ×÷ŠŌ£¬²»ŅĖŹ¹²Ł×÷±äµĆ¹żÓŚø“ŌÓ£© ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com