
��������(H2O2)��һ����ɫ��Һ��,����ˮ��Һ�׳�˫��ˮ,��������,����������������ɱ������Ư���ȡ�
(1)����˵����ȷ���� ��
| A��������������м��м��Լ����зǼ��Լ� |
| B��H2O2��H2O��Ϊͬ�������� |
| C��34 g H2O2�к��е���������ΪNA |
| D��ʵ���ҿ������ù���������ȡ���� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ۻ�(As4S4)�ʹƻ�(As2S3)����ȡ�����Ҫ����ԭ�ϣ���������Ȼ���й����������������������գ�
(1)As2S3��SnCl2�������з�Ӧת��ΪAs4S4��SnCl4���ų�H2S���塣��As2S3��SnCl2������ȫ��Ӧ��As2S3��SnCl2�����ʵ���֮��Ϊ ��
(2)������Ӧ�е��������� ����Ӧ������������� ���ա�
(3)As2S3��HNO3�����·�Ӧ��
As2S3��10H����10NO3��=2H3AsO4��3S��10NO2����2H2O������2 mol H3 AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ �������÷�Ӧ��Ƴ�һԭ��أ���NO2Ӧ���� (�������������)�����ݳ���
(4)����Ӧ����NO2��11.2 L O2(��״��)��Ϻ���ˮ����ȫ��ת����ŨHNO3��Ȼ���������̼��Ӧ����������CO2���� (ѡ����)��
a����0.5 mol b������0.5 mol
c������0.5 mol d����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɻ�ͭ��(��Ҫ�ɷ���CuFeS2)���ƾ�ͭ�Ĺ�������ʾ��ͼ���£�
��1���ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1000�����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ�������ֵͼ�����Ļ�ѧʽ�ֱ�Ϊ________��_______���ڷ�Ӧ�����л���һ����Fe������ת��Ϊ�ͼ�������仯ѧ��Ӧ����ʽΪ____________��
��2����ͭ(Cu2S��FeS�����ۺ϶���)��Cu���ϵ͡�ת¯�У�����ͭ���ۼ�(ʯӢɰ)��1200�����Ҵ���������д�������ͭ�е�Cu2S��������Cu2O����ÿ��1mol�����μӷ�Ӧ������������������ʵ���Ϊ_________�����ɵ�Cu2O��Cu2S��Ӧ�����Ƶú�Cu���ϸߵĴ�ͭ��
��3����ͭ�ĵ�⾫����ͼ��ʾ���ڴ�ͭ�ĵ������У�cΪ��ͭ�壬 ��a��Ӧ���ӵ�Դ��_____��(���������)������ͭ�к���Au��Ag��Fe���ʣ����������c�缫�Ϸ�����Ӧ�ķ���ʽ��__________��
��4������Ӧ�����Ը��������Һ�ζ����ⶨ��Ӧ����Һ����Ԫ�صĺ������ζ�ʱ�����ü�ʽ�ζ���ʢ�����Ը��������Һ��ԭ����____________��������Ӧ�����ӷ���ʽΪ_____________���ζ�ʱ����ƿ�е���Һ��Ӵ������������Ԫ�صĺ�����____(�ƫ�ߡ���ƫ�͡�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ƴ���ͭ��ˮ�к���CN����Cr2O72-����Ҫ������������ŷš��ó��ⶨ�������̽��з�ˮ������
�ش��������⣺
��1������������ˮ��������Ҫʹ�õķ�����________��
��2�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ______________________________
__________________________________��
��3��������У�ÿ����0.4 mol Cr2O72-ʱת�Ƶ���2.4 mol���÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
��4��ȡ��������ˮ�����Թ��У�����NaOH��Һ���۲쵽����ɫ�������ɣ��ټ�Na2S��Һ����ɫ����ת���ɺ�ɫ��������ʹ�û�ѧ��������ֽ��Ͳ����������ԭ��
________________________________________________________________________��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4��7H2O����Cr2O72-��ԭ��Cr3��������pH��Fe��Crת�����൱��Fe��[FeCr]O4(�������壬�������ֱ�ʾԪ�ؼ�̬)�ij���������1 mol Cr2O72-�������a mol FeSO4��7H2O�����н�����ȷ����________��
| A��x��0.5��a��8�������� | B��x��0.5��a��10 |
| C��x��1.5��a��8 | D��x��1.5��a��10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(13��)�������Ļ������ڹ�ҵ�������ճ������ж��й㷺����;��
��1���ڶ������У�������������������Ӧ�ų����������и�ֽ�÷�Ӧ�Ļ�ѧ����ʽΪ�ߣߡ�
��2����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H��+468.2 kJ��mol-1
C(s)+O2(g)��CO2(g) ��H="-393.5" kJ��mol-1��
��Fe(s)��O2 (g)��Ӧ����Fe2 O3 (s)���Ȼ�ѧ����ʽΪ��_____________________��
��3������KMnO4��Һ�ζ�Fe2+��Ũ�ȣ���Ӧ�����ӷ���ʽ���£�5Fe2����MnO4����8H����5Fe3����Mn2����4H2O
��KMnO4��ҺӦʢ���ڣߣߣߣߣߵζ����У�
���жϴﵽ�ζ��յ�������ǣߣߣߣߣߣ�
���������ữ��0.020 00 mol��L-1��KMnO4��Һ�ζ�ijFeSO4��Һ���յ㣬ʵ�����ݼ�¼���±���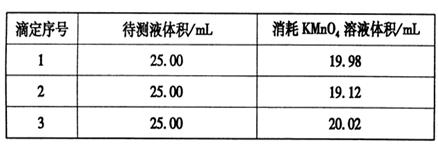
��������ݲ����㣬��FeSO4��Һ�����ʵ���Ũ��Ϊ�ߣߣߣߣߡ�
��4���������ײ���ZnFe2Ox�������ڳ�ȥ��ҵ�����е�ijЩ�������ȡ�²��Ϻͳ�ȥ������ת����ϵ����ͼ��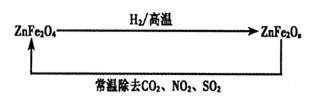
����֪ZnFe2O4��H2��Ӧ�����ʵ���֮��Ϊ2:1����ZnFe2Ox��x=�ߣߣߣߣߣ�
����ZnFe2Ox��ȥSO2�Ĺ����У��������ǣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ھ�������������ˮ��������������ҵ�������Ư����
��1���������������������ҿ������������е��ʷ�Ӧ���磺6Ag��s����O3��g��=3Ag2O��s������H����235.8 kJ��mol��1����֪2Ag2O��s��=4Ag��s����O2��g�� ��H����62.2 kJ��mol��1�������·�Ӧ2O3��g��=3O2��g���Ħ�H��________��
��2����ƽ���淴Ӧ�Ļ�ѧ����ʽ���������ʵĻ�ѧ������������Ӧ�ķ����ڣ���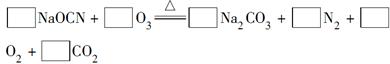
��3����ѧ��P.Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в������缫��ӦʽΪ3H2O��6e��=O3����6H�������������ܽ���ˮ�е��������ɹ������⣬��缫��ӦʽΪ_______________________��
��4�������г����ļ�ⷽ���ǽ���������ͨ������KI������Һ������Һ����ɫ����˵�������к���O3����֪O3��KI��Һ��Ӧ�������ֵ��ʣ���÷�Ӧ�����ӷ���ʽΪ_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���뽫�����������ʣ�KBr��Br2��I2��KI��K2SO4�ֱ��������к����ϣ����һ��δ��ƽ�Ļ�ѧ����ʽ��
KBrO3��________��H2SO4�D��________��________��________��________��H2O��
��2������û�ѧ����ʽ��I2��KBr�Ļ�ѧ�������ֱ���8��1����
��Br2�Ļ�ѧ��������________��
���뽫��Ӧ��Ļ�ѧʽ����ƽ��Ļ�ѧ����������������Ӧ��λ���У�
________KBrO3��________��________H2SO4�D��������
����ת��10 mol���ӣ���Ӧ������I2�����ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�⻯��ͭ(CuH)��һ���������ʣ���CuSO4��Һ�͡���һ���ʡ���40��50��ʱ��Ӧ����������CuH���е������У����ȶ����ֽ⣻����������ȼ�գ���ϡ���ᷴӦ���������壻Cu�������������·����ķ�Ӧ�ǣ�2Cu��=Cu2����Cu��
����������Ϣ������Լ������յĻ�ѧ֪ʶ���ش��������⣺
(1)��CuSO4��Һ�͡���һ���ʡ���CuH�ķ�Ӧ�У���������ԭ�۵�������⡰��һ���ʡ��ڷ�Ӧ����______(�����������ԭ����)��
(2)д��CuH��������ȼ�յĻ�ѧ��Ӧ����ʽ��________________________��
(3)CuH�ܽ���ϡ���������ɵ�������______(�ѧʽ)��
(4)�����CuH�ܽ���������ϡ���������ɵ�����ֻ��NO����д��CuH�ܽ�������ϡ�����з�Ӧ�����ӷ���ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ߡ������־���ҹ��ĺ�����ҵ�������µ�ƪ�¡�
��1���������ʱ�������������ľ���Ħ�����������¡�Ϊ�˷�ֹ����¶ȹ��ߣ��ڻ��һ��Ϳ��һ�������Ϳ�ϣ���Ϳ�ϵ���������ܵ��� ��
| A���ڸ����²��ڻ� | B���ڸ����¿ɷֽ����� |
| C���ڳ����¾ͷֽ����� | D����Ϳ�ϲ����ܷ����ֽ� |

 CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1
CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1 CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1
CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1 CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com