
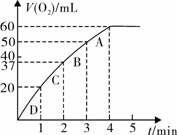
��11�֣�����0.10 mol MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��
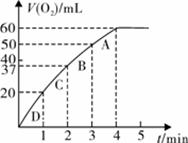
��д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ ����2�֣�
��ʵ��ʱ�ų������������� mL��
�۷ų�1/3��������ʱ��Ϊ min��
�ܷ�Ӧ�ų�3/4��������ʱ��ԼΪ min��
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ_____��____��____��____����2�֣�
���ͷ�Ӧ���ʱ仯��ԭ�� ����2�֣�
����H2O2�ij�ʼ���ʵ���Ũ��_____________�����뱣����λ��Ч���֣���2�֣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ���ض��и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
�����������ͿƼ��������ش����á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺ 
��1�����Է�������ͼ��ͨ���۲� �����ԱȽϵó����ۡ�ͬѧX�۲������֧�Թܲ������ݵĿ������ɴ˵ó�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч���������________(���������������)��������______________________��
��2��������������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������ ���� ��
��3������0.10 mol MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��
��ʵ��ʱ�ų������������� mL��
�ڷų�1��3��������ʱ��Ϊ min��
�ۼ���H2O2�ij�ʼ���ʵ���Ũ��_____________�� ���뱣����λ��Ч���֣�
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ_____��____��____��____��
�� ���ܷ͢�Ӧ���ʱ仯��ԭ�� ___ _____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

��1�����Է�������ͼ��ͨ���۲� �����ԱȽϵó����ۡ���ͬѧ�����Cu SO4��ΪCuCl2��Ϊ�������������� ������������
��2��������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ������������ �� �� ��
��3������0.10 mol MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��
��ʵ��ʱ�ų������������� mL��
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ_____��____��____��____��
�۽��ͷ�Ӧ���ʱ仯��ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���и�һ�ڶ�ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
Ϊ�Ƚ�Fe2+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش��������⣺

��1�����Է�������ͼ��ͨ���۲� ���ԱȽϵó����ۣ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
��2��������������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ�������� ��
��3��ijͬѧҪ������װ�òⶨijH2O2��Һ�����ʵ���Ũ�ȡ�����ƿ�м���0.10 mol MnO2��ĩ�����50 mL ��H2O2��Һ���ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��

��д��H2O2��MnO2�����·�����Ӧ�Ļ�ѧ����ʽ ��
��ʵ���зų������������� mL��
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ > > > ��
�ܼ���H2O2�ij�ʼ�����ʵ���Ũ�� �����������2λС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�߿�������-�����Ԫ�ؼ��仯���� ���ͣ�ʵ����
Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺


��
��1�����Է�������ͼ��ͨ���۲� �����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3 ��Ϊ���������������������� ����
��2��������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ������װ�������Եķ�����
��ʵ������Ҫ������������ ��
��3������0.10 mol MnO2��ĩ��50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ����ʾ
�� д��H2O2��MnO2�����·�����Ӧ�Ļ�ѧ����ʽ ������
�ڼ���H2O2�ij�ʼ���ʵ���Ũ��_____________�� ���뱣����λ��Ч���֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com