
 CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�| ʵ���� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2 mL�Ҵ���2 mL���ᡢ1 mL 18 mol��L��1Ũ���� | ����̼������Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6mL 3 mol��L��1���� | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��19.6% | B��22% | C��36.4% | D��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
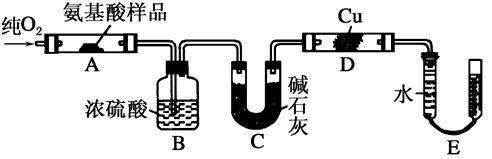
| A������CO2��������� |
| B������H2O������ |
| C��ͨ��O2����� |
| D�����������Է������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����к��еı������ʿ�ͨ������������ˮ����˵ķ�������ȥ |
| B������ϩ��������8��̼ԭ����ͬһ��ƽ�� |
| C������������ϩ�;���ϩ��ȫȼ�գ������Ķ�����̼������֮��Ϊ1��1 |
| D������ʽΪC5H10O2�ҿ�������������Һ��Ӧ���л���������14�֣������������칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ҵ������� | B����ϩ����ϩ | C���������� | D�����顢�״� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
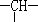 ��a����CH3������Ϊ��OH�����ǻ��ĸ���Ϊ (����)
��a����CH3������Ϊ��OH�����ǻ��ĸ���Ϊ (����)| A��2n��3m��a | B��m��2��a | C��n��m��a | D��m��2n��2��a |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com