
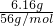 ��116x+89y=12.49
��116x+89y=12.49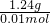 =124g/mol��
=124g/mol��

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ��������⻯ѧ�Ծ� ���ͣ������
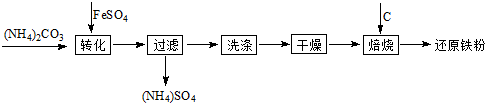
��ԭ�����Ƿ�ĩұ��ҵ����Ҫԭ�ϣ����������Ѱĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£�
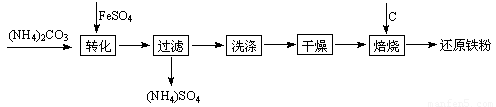
�Ÿ��������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3��nH2O����������ΪFeOOH���仯ѧ����ʽΪ ��
��ȡ������FeCO3��Ʒ12.49 g������600�棬������Ϊ8.00 g�������������յõ�Fe 6.16 g����600�����Ŀ������Ϊ ������FeCO3��Ʒ��FeCO3��FeOOH��������
������28.12 g��ԭ���ۣ�������FexC�����������м��ȣ��õ���״���µ�CO2 224 mL������ͬ�����Ļ�ԭ�������������ᷴӦ���õ���״���µ�H2 10.752 L������FexC�Ļ�ѧʽ�������軹ԭ���۽����������ʣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ԭ�����Ƿ�ĩұ��ҵ����Ҫԭ�ϣ����������Ѱĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£�
�Ÿ��������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3��nH2O����������ΪFeOOH���仯ѧ����ʽΪ ��
��ȡ������FeCO3��Ʒ12.49 g������600�棬������Ϊ8.00 g�������������յõ�Fe 6.16 g����600�����Ŀ������Ϊ ������FeCO3��Ʒ��FeCO3��FeOOH��������
������28.12 g��ԭ���ۣ�������FexC�����������м��ȣ��õ���״���µ�CO2 224mL������ͬ�����Ļ�ԭ�������������ᷴӦ���õ���״���µ�H210.752 L������FexC�Ļ�ѧʽ�������軹ԭ���۽����������ʣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����ѧ������ѧ��������⻯ѧ�Ծ� ���ͣ������
��ԭ�����Ƿ�ĩұ��ҵ����Ҫԭ�ϣ����������� �ĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£�
�ĸ���Ʒ�̷��Ʊ���ԭ���۵Ĺ�ҵ�������£� 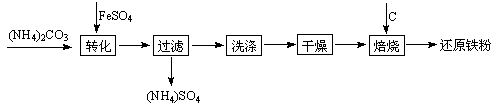
�Ÿ��������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3��nH2O����������ΪFeOOH���仯ѧ����ʽΪ ��
��ȡ������FeCO3��Ʒ12.49 g������600�棬������Ϊ8.00 g�������������յõ�Fe 6.16 g����600�����Ŀ������Ϊ ������FeCO3��Ʒ��FeCO3��FeOOH��������
������28.12 g��ԭ���ۣ�������FexC�����������м��ȣ��õ���״���µ�CO2 224 mL������ͬ�����Ļ�ԭ�������������ᷴӦ���õ���״���µ�H2 10.752 L������FexC�Ļ�ѧʽ�������軹ԭ���۽����������ʣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com