
ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�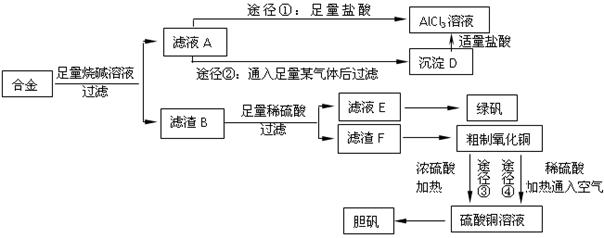
�ش��������⣺
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ ��������Ϊ�Ͻ����ռ���Һ�γ���ԭ��أ�����Ϊԭ��ظ����������� ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�������� ������ʵ�鷽���ദ�����˹��˲������������õ��IJ��������� �Ͳ����������в������������� ��
��3���ô�������ͭͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ���: �� ��
��4��ͨ��;����ʵ���ô�������ͭ��ȡ������������е�ʵ��������裺���ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ������С�����ͨ���������������Ϊ �������ӷ���ʽ��ʾ����
��5���ڲⶨ���õ�����CuSO4��xH2O���нᾧˮxֵ��ʵ������У������������ٽ��� �Ρ����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯
e������ʱ��������ɽ����� f��������������δ�����ʪ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
�ش��������⣺
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ ��������Ϊ�Ͻ����ռ���Һ�γ���ԭ��أ�����Ϊԭ��ظ����������� ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�������� ������ʵ�鷽���ദ�����˹��˲������������õ��IJ��������� �Ͳ����������в������������� ��
��3���ô�������ͭͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ���: �� ��
��4��ͨ��;����ʵ���ô�������ͭ��ȡ������������е�ʵ��������裺���ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ������С�����ͨ���������������Ϊ �������ӷ���ʽ��ʾ����
��5���ڲⶨ���õ�����CuSO4��xH2O���нᾧˮxֵ��ʵ������У������������ٽ��� �Ρ����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯
e������ʱ��������ɽ����� f��������������δ�����ʪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
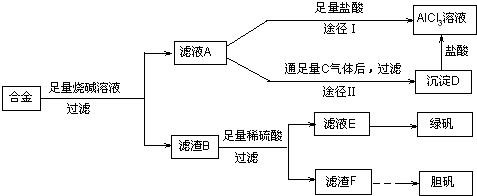
��18�֣�ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����[FeSO4��7H2O]�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
�ش��������⣺
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ ��
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�������� ������ʵ�鷽���ദ�����˹��˲������������õ��IJ��������� �Ͳ����������в������������� ��
��3��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��� �� ��
��4��ͨ��;������ȡ������������е�ʵ��������裺�����ᡢ����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ������С�����ͨ���������������Ϊ �������ӷ���ʽ��ʾ����
![]() ��5���ⶨ���õ�����CuSO4��xH2O����xֵ��ʵ�鷽������������ͭ�����е�ˮ�õ���ɫ����ˮ����ͭ����ȴ��������˱仯�Ļ�ѧ����ʽΪ��CuSO4��xH2O===CuSO4+xH2O �����¶ȹ��ߣ�CuSO4������ֽ�ΪCuO��SO3���ڴ�ʵ������У������������ٽ��� �Ρ����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
��5���ⶨ���õ�����CuSO4��xH2O����xֵ��ʵ�鷽������������ͭ�����е�ˮ�õ���ɫ����ˮ����ͭ����ȴ��������˱仯�Ļ�ѧ����ʽΪ��CuSO4��xH2O===CuSO4+xH2O �����¶ȹ��ߣ�CuSO4������ֽ�ΪCuO��SO3���ڴ�ʵ������У������������ٽ��� �Ρ����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
a�������¶ȹ��� b������ʱ��������ɽ�����
c�����Ⱥ���ڿ�������ȴ d��������������δ�����ʪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ����һ�и���Ѻ�⣨�����ۺϣ���ѧ���� ���ͣ������
��16�֣�I.����a��e����ѧ��ѧʵ���г����ļ��ֶ���������
a.��Ͳ b.����ƿ c.�ζ��� d.������ƽ e.�¶ȼ�
��ʹ������ƿ�ĵ�һ��������___________________________________��
������������������500mL 2mol��L��1��NaCl��Һ����ȱ�ٵIJ���������_________��
���������������ⶨ�к��ȣ���ȱ�ٵIJ�������Ϊ�ձ���__________��
�������������������к͵ζ�����ȱ�ٵ�������__________��
��ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�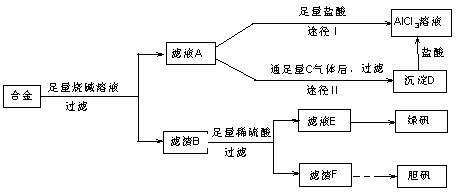
�Իش���������: w.w.w.k.&s.5*u.c.om
��1���õ��������У���ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������
��
��2������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ�������� ��������
 ��
��
��3������ҺE�еõ��̷������ʵ������� ��
��4��д��������F�Ʊ�����������йػ�ѧ����ʽ
��
��5����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ������ ��������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ӱ�ʡ����Ѻ�⣨�����ۺϣ���ѧ���� ���ͣ������
��16�֣�I.����a��e����ѧ��ѧʵ���г����ļ��ֶ���������
a.��Ͳ b.����ƿ c.�ζ��� d.������ƽ e.�¶ȼ�
��ʹ������ƿ�ĵ�һ��������___________________________________��
������������������500mL 2mol��L��1��NaCl��Һ����ȱ�ٵIJ���������_________��
���������������ⶨ�к��ȣ���ȱ�ٵIJ�������Ϊ�ձ���__________��
�������������������к͵ζ�����ȱ�ٵ�������__________��
�� ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
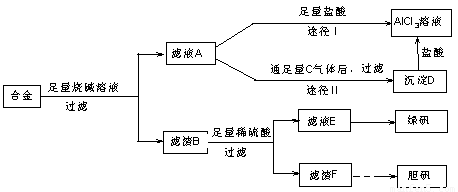
�Իش���������:
��1���õ��������У���ֽ������̨����Ȧ���ձ�����Ҫ����IJ���������
��
��2������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ�������� ��������
��
��3������ҺE�еõ��̷������ʵ������� ��
��4��д��������F�Ʊ�����������йػ�ѧ����ʽ
��
��5����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ������ ��������
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com