
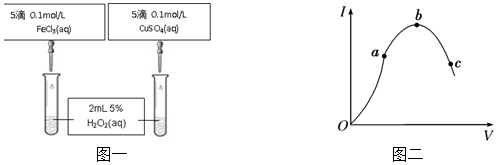
·ÖĪö £Ø¢ń£©²śÉśĘųÅŻµÄæģĀżæÉŅŌ¶ØŠŌČ·¶ØĖ«ŃõĖ®µÄ·Ö½āĖŁĀŹ“󊔣¬±Č½ĻFe3+ŗĶCu2+¶ŌH2O2·Ö½āµÄ“߻Ɗ§¹ū£¬ŅŖČĆŃ”ŌńµÄŹŌ¼ĮFe3+ŗĶCu2+ÖŠµÄŅõĄė×ÓÖÖĄąĻąĶ¬²ÅÓŠŅāŅ壻
£Ø¢ņ£©£Ø1£©ČÜŅŗµÄµ¼µēŠŌÓėĄė×ÓÅضČÓŠ¹Ų£¬Ąė×ÓÅضČŌ½“󣬵¼µēŠŌŌ½Ē棻
£Ø2£©µ¼µēÄÜĮ¦Ō½Ē棬Ąė×ÓÅضČŌ½“ó£¬ĒāĄė×ÓÅضČŌ½“󣬣»
£Ø3£©ČÜŅŗŌ½Ļ”£¬Ō½“Ł½ų“×ĖįµēĄė£¬ŌņČÜŅŗÖŠĒāĄė×ÓµÄĪļÖŹµÄĮæŌ½“󣬵ēĄė³Ģ¶ČŌ½“ó£»
£Ø4£©Ź¹c£ØCH3COO-£©Ōö“ó£¬ČÜŅŗc£ØH+£©¼õŠ”£¬æÉŅŌ¼ÓÉŁĮæNaOH¹ĢĢ唢¼ÓÉŁĮæNa2CO3¹ĢĢ唢¼ÓČėZn”¢MgµČ½šŹō£®
½ā“š ½ā£ŗ£Ø¢ń£©æÉŅŌĶعż¹Ū²ģ²śÉśĘųÅŻµÄæģĀżĄ“¶ØŠŌ±Č½Ļ±Č½ĻFe3+ŗĶCu2+¶ŌH2O2·Ö½āµÄ“߻Ɗ§¹ū£¬½«CuSO4øÄĪŖCuCl2øüĪŖŗĻĄķ£¬ÕāŃłFe3+ŗĶCu2+ÖŠµÄŅõĄė×ÓÖÖĄąĻąĶ¬£¬æÉŅŌÅųżŅņŅõĄė×ӵIJ»Ķ¬æÉÄÜ“ųĄ“µÄÓ°Ļģ£¬»¹æÉŅŌ°ŃĀČ»ÆĢśøÄĪŖĮņĖįĢś£¬
¹Ź“š°øĪŖ£ŗ²śÉśĘųÅŻµÄæģĀż£»æŲÖĘŅõĄė×ÓĻąĶ¬£¬ÅųżŅõĄė×ÓµÄøÉČÅ£»½«ĀČ»ÆĢśøÄĪŖĮņĖįĢś£»
£ØII£©£Ø1£©ČÜŅŗµÄµ¼µēŠŌÓėĄė×ÓÅضČÓŠ¹Ų£¬Ąė×ÓÅضČŌ½“󣬵¼µēŠŌŌ½Ē棬±ł“×Ėį֊ƻӊ×ŌÓÉŅĘ¶ÆµÄĄė×Ó£¬ĖłŅŌ±ł“×Ėį²»µ¼µē£¬
¹Ź“š°øĪŖ£ŗŌŚ”°O”±µć“¦“×ĖįĪ“µēĄė£¬ĪŽĄė×Ó“ęŌŚ£»
£Ø2£©ÓÉÓŚµ¼µēÄÜĮ¦Ō½Ē棬ČÜŅŗÖŠĄė×ÓÅضČŌ½“ó£¬ĒāĄė×ÓÅضČŌ½“ó£¬
¹Ź“š°øĪŖ£ŗc£¼a£¼b£»
£Ø3£©ČÜŅŗŌ½Ļ”£¬Ō½“Ł½ų“×ĖįµēĄė£¬ŌņČÜŅŗÖŠĒāĄė×ÓµÄĪļÖŹµÄĮæŌ½“󣬵ēĄė³Ģ¶ČŌ½“ó£¬ĖłŅŌµēĄė³Ģ¶ČµÄŹĒc£¬
¹Ź“š°øĪŖ£ŗc£»
£Ø4£©ČōŹ¹c£ØCH3COO-£©Ōö“ó£¬ČÜŅŗc£ØH+£©¼õŠ”£¬æɲÉČ”µÄ“ėŹ©ŹĒ£ŗ¢Ł¼ÓÉŁĮæNaOH¹ĢĢå””¢Ś¼ÓÉŁĮæNa2CO3¹ĢĢå””¢Ū¼ÓČėZn”¢MgµČ½šŹō£¬
¹Ź“š°øĪŖ£ŗ¼ÓÉŁĮæNaOH¹ĢĢ壻¼ÓÉŁĮæNa2CO3¹ĢĢå»ņ¼ÓČėZn”¢MgµČ½šŹō£®
µćĘĄ ±¾Ģāæ¼²é“߻ƼĮµÄ×÷ÓĆ”¢µē½āÖŹµÄµēĄė£¬ĢāÄæÄѶČÖŠµČ£¬¼ÓĖ®Ļ”ŹĶ“×Ėį£¬ÄÜ“Ł½ų“×ĖįµēĄė£¬µ«ČÜŅŗÖŠ“×ĖįøłĄė×ÓŌö“óµÄĮæŌ¶Ō¶Š”ÓŚĖ®Ģå»żŌö“óµÄĮ棬ĖłŅŌ“×ĖįøłĄė×ÓÅØ¶Č¼õŠ”£¬ĪŖŅדķµć£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³ĪĒåµÄŹÆ»ŅĖ®ÓėĻ”ĻõĖį·“Ó¦£ŗOH-+H+ØTH2O | |
| B£® | ĒāŃõ»Æ±µČÜŅŗÓėĮņĖįµÄ·“Ó¦£ŗOH${\;}^{{-}^{\;}}$+H+ØTH2O | |
| C£® | ĢśŗĶĻ”ĮņĖį·“Ó¦£ŗ2Fe+6H+ØT2Fe3++3H2”ü | |
| D£® | “×ĖįČÜŅŗÓėĢ¼ĖįøĘ·“Ó¦£ŗCaCO3+2H+ØTCa2++CO2”ü+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĖüĆĒĖłŗ¬ŃõŌ×ÓŹżÄæÖ®±ČĪŖ2£ŗ3 | B£® | ĖüĆĒµÄĢå»żÖ®±ČĪŖ1£ŗ1 | ||
| C£® | ĖüĆĒĖłŗ¬Ō×ÓŹżÄæÖ®±ČĪŖ3£ŗ4 | D£® | ĖüĆĒµÄ·Ö×ÓŹżÄæÖ®±ČĪŖ1£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
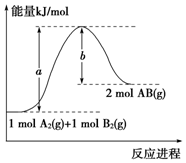
| A£® | ĆæÉś³É2·Ö×ÓABĪüŹÕb kJČČĮæ | |
| B£® | øĆ·“Ó¦¹ż³ĢµÄ»ī»ÆÄÜĪŖb kJ•mol-1 | |
| C£® | ¶ĻĮŃ1 mol A-AŗĶ1 mol B-B¼ü£¬·Å³öa kJÄÜĮæ | |
| D£® | øĆ·“Ó¦µÄ·“Ó¦ČČ”÷H=+£Øa-b£© kJ•mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ag+”¢K+”¢NO3-”¢Cl- | B£® | Mg2+”¢Na+”¢Cl-”¢SO42- | ||
| C£® | Ca2+”¢Mg2+”¢OH-”¢Cl- | D£® | H+”¢Na+”¢CO32-”¢SO42- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com