
 HCO3��+OH-���������˵������Fe2O3��
HCO3��+OH-���������˵������Fe2O3�� Fe��OH��3 + 3H+��2Fe��OH��3=��=Fe2O3+3H2O�� ��� ��ֹFe2+��������
Fe��OH��3 + 3H+��2Fe��OH��3=��=Fe2O3+3H2O�� ��� ��ֹFe2+��������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��S(s) + O2(g) ��SO2(g)��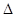 H=�� H=��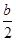 kJ/mol kJ/mol |
B��S(g) + O2(g) ��SO2(g)��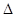 H=�� H=��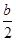 kJ/mol kJ/mol |
C��S(s) + O2(g) ��SO2(g)��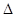 H=��(2a- H=��(2a-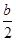 )kJ/mol )kJ/mol |
D��S(s) + O2(g) ��SO2(g)��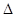 H=��(b-a)kJ/mol H=��(b-a)kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��+184.6kJ��mol-1 | B���D92.3kJ��mol-1 |
| C��+92.3kJ | D��+92.3kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���
2NO(g) DH��a kJ��mol��1��ƽ�ⳣ��K���±���| �¶�/�� | 1538 | 1760 | 2404 |
| ƽ�ⳣ��K | 0.86��10��4 | 2.6��10��4 | 64��10��4 |
 2NO(g)�ﵽƽ��ʱN2��Ũ��Ϊ �������¶��²�����O2��NO�ķ�Ӧ��������������λ��Ч���֣�
2NO(g)�ﵽƽ��ʱN2��Ũ��Ϊ �������¶��²�����O2��NO�ķ�Ӧ��������������λ��Ч���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��4Q1+0.5Q2 | B��4Q1+Q2+10Q3 | C��4Q1+2Q2 | D��4Q1+0.5Q2+9Q3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��488.3 | B����244.15 | C��244.15 | D����488.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��+172.5 kJ/mol | B��-172.5 kJ/mol | C��+110.5kJ/mol | D��-110.5kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com