
»Ų“šĻĀĮŠÓŠ¹Ų³£ŹżµÄĪŹĢā
£Ø1£©KwµÄŹżŃ§±ķ“ļŹ½ŹĒKw= £¬ĪĀ¶ČÉżøßKwµÄ±ä»ÆĒéæöŹĒ £ØŃ”Ģī”°±ä“ó”±”¢”°±ä
Š””±»ņ”°²»±ä”±£©”£
£Ø2£©KĶس£±ķŹ¾»ÆŃ§Ę½ŗā³£Źż£¬KÖµŌ½“ó±ķŹ¾øĆ·“Ó¦½ųŠŠµĆŌ½ £¬¶ŌÓŚ·“Ó¦2NO2(g)  N2O4(g)£¬Ęä»ÆŃ§Ę½ŗā³£KµÄŹżŃ§±ķ“ļŹ½ĪŖ
N2O4(g)£¬Ęä»ÆŃ§Ę½ŗā³£KµÄŹżŃ§±ķ“ļŹ½ĪŖ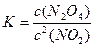 £¬Ź½ÖŠc2(NO2)±ķŹ¾µÄŅāŅåŹĒ ”£
£¬Ź½ÖŠc2(NO2)±ķŹ¾µÄŅāŅåŹĒ ”£
£Ø3£©KaĶس£±ķŹ¾ČõĖįµÄµēĄėĘ½ŗā³£Źż£¬¦ĮĶس£³ĘĪŖµēĄė¶Č”£¶ŌÓŚÄ³Ņ»ŌŖČõĖį£¬µ±ÓĆÕōĮóĖ®Ļ”ŹĶøĆĖįŹ±£¬ČÜŅŗŌ½Ļ”£¬KaµÄ±ä»ÆĒéæöŹĒ £ØŃ”Ģī”°±ä“ó”±”¢”°±äŠ””±»ņ
”°²»±ä”±£©£¬¦ĮµÄ±ä»ÆĒéæöŹĒ £ØŃ”Ģī”°±ä“ó”±”¢”°±äŠ””±»ņ”°²»±ä”±£©”£
 ÖŠæ¼Ąū½£ÖŠæ¼ŹŌ¾ķ»ć±ąĻµĮŠ“š°ø
ÖŠæ¼Ąū½£ÖŠæ¼ŹŌ¾ķ»ć±ąĻµĮŠ“š°ø ½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø
½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø »ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø
»ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| 16 |
| 3 |
| ČŻĘ÷ | ČŻĘ÷1 | ČŻĘ÷2 | ČŻĘ÷3 |
| ·“Ó¦ĪļĶ¶ČėĮæ£ØŹ¼Ģ¬£© | 1molCO2”¢3molH2 | 0.5molCO2”¢1.5molH2 | 1molCH3OH”¢1molH2O |
| CH3OHµÄĘ½ŗāÅضČ/mol?L-1 | c1 | c2 | c3 |
| Ę½ŗāŹ±ĢåĻµŃ¹Ēæ/Pa | p1 | p2 | p3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌÓŚČõĖį£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ“ļµ½µēĄėĘ½ŗāŹ±£¬ø÷Ī¢Į£µÄÅØ¶Č“ęŌŚŅ»ÖÖ¶ØĮæµÄ¹ŲĻµ£®ĻĀ±ķŹĒ25”ꏱ¼øÖÖ³£¼ūČõĖįµÄµēĄėĘ½ŗā³£Źż
| Ėį | µēĄė·½³ĢŹ½ | µēĄėĘ½ŗā³£ŹżK |
| | | |
| | | |
| | | |
| | | |
»Ų“šĻĀĮŠø÷ĪŹ£ŗ
£Ø1£©KÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬µ±ĪĀ¶ČÉżøߏ±£¬KÖµ________£ØĢī”°Ōö“ó”±”¢”°¼õŠ””±”¢”°²»±ä”±£©£®
£Ø2£©ŌŚĪĀ¶ČĻąĶ¬Ź±£¬ø÷ČõĖįµÄKÖµ²»Ķ¬£¬ÄĒĆ“KÖµµÄ“óŠ”ÓėĖįŠŌµÄĻą¶ŌĒæČõÓŠŗĪ¹ŲĻµ?__________________£®
£Ø3£©Čō°ŃCH3COOH”¢H2CO3”¢HCO3-”¢H2S”¢HS-”¢H3PO4”¢H2PO4-”¢HPO42-¶¼æ“×÷ŹĒĖį£¬ĘäÖŠĖįŠŌ×īĒæµÄŹĒ_________£¬×īČõµÄŹĒ________£®
£Ø4£©¶ąŌŖČõĖįŹĒ·Ö²½µēĄėµÄ£¬ĆæŅ»²½¶¼ÓŠĻąÓ¦µÄµēĄėĘ½ŗā³£Źż£®¶ŌÓŚĶ¬Ņ»ÖÖ¶ąŌŖČõĖįµÄK1”¢K2”¢K3Ö®¼ä“ęŌŚ×ÅŹżĮæÉĻµÄ¹ęĀÉ£¬¶ŌÓŚH3PO4“Ė¹ęĀÉŹĒ________________£¬²śÉś“Ė¹ęĀɵÄŌŅņŹĒ_________________________£®
![]() £Ø5£©µēĄėĘ½ŗā³£ŹżŹĒÓĆŹµŃéµÄ·½·Ø²ā¶Ø³öĄ“µÄ£®ĻÖŅŃ¾²āµĆijĪĀ¶ČĻĀ NH3∙H2OČÜŅŗÖŠ“ęŌŚČēĻĀ·“Ó¦£ŗNH3∙H2O NH4++OH- ŅŃÖŖ0.10 mol”¤L-1NH3∙H2OČÜŅŗÖŠ£¬“ļµ½Ę½ŗāŹ±£¬CĘ½ŗā£ØOH-£©=4.2 ”Į 10-3mol”¤L-1£¬CĘ½ŗā£ØNH3∙H2O£©”ÖCĘšŹ¼£ØNH3∙H2O£©£¬Ė®µÄµēĄėæÉŗöĀŌ²»¼Ę;
£Ø5£©µēĄėĘ½ŗā³£ŹżŹĒÓĆŹµŃéµÄ·½·Ø²ā¶Ø³öĄ“µÄ£®ĻÖŅŃ¾²āµĆijĪĀ¶ČĻĀ NH3∙H2OČÜŅŗÖŠ“ęŌŚČēĻĀ·“Ó¦£ŗNH3∙H2O NH4++OH- ŅŃÖŖ0.10 mol”¤L-1NH3∙H2OČÜŅŗÖŠ£¬“ļµ½Ę½ŗāŹ±£¬CĘ½ŗā£ØOH-£©=4.2 ”Į 10-3mol”¤L-1£¬CĘ½ŗā£ØNH3∙H2O£©”ÖCĘšŹ¼£ØNH3∙H2O£©£¬Ė®µÄµēĄėæÉŗöĀŌ²»¼Ę;
¢ŁÓĆpHŹŌÖ½²āĮæČÜŅŗµÄpHÖµ£¬¼“æÉĒóµĆCĘ½ŗā£ØOH-£©£¬²ā¶ØČÜŅŗpHÖµµÄ²Ł×÷ŹĒ______________”£
¢Ś²āĮæCĘ½ŗā£ØNH3∙H2O£©µÄ·½·Ø×īŗĆÓĆ_____________·Ø£ØĢī·½·ØĆū³Ę£©
¢ŪĒó“ĖĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżK.(Š“³ö¼ĘĖć¹ż³Ģ£¬¼ĘĖć½į¹ū±£Įō2Ī»ÓŠŠ§Źż×Ö)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¹ć¶«Ź”Ö“ŠÅ֊ѧø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
¶ŌÓŚČõĖį£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ“ļµ½µēĄėĘ½ŗāŹ±£¬ø÷Ī¢Į£µÄÅØ¶Č“ęŌŚŅ»ÖÖ¶ØĮæµÄ¹ŲĻµ£®ĻĀ±ķŹĒ25”ꏱ¼øÖÖ³£¼ūČõĖįµÄµēĄėĘ½ŗā³£Źż
| Ėį | µēĄė·½³ĢŹ½ | µēĄėĘ½ŗā³£ŹżK |
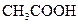 |   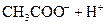 |  |
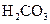 |  | 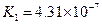 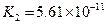 |
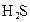 | 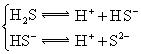 | 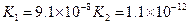 |
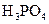 | 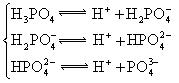 | 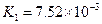 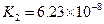 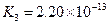 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ź”Ģ©°²ŹŠøßČżµŚ¶žĀÖø“Ļ°ÖŹĮæ¼ģ²āĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
½üÄźĄ“£¬ŅŌĢģČ»ĘųµČĪŖŌĮĻŗĻ³É¼×“¼µÄÄŃĢā±»Ņ»Ņ»¹„æĖ£¬¼«“óµŲ“Ł½ųĮĖ¼×“¼»ÆѧµÄ·¢Õ¹”£
£Ø1£©ÓėĢæŗĶĖ®ÕōĘųµÄ·“Ó¦ĻąĖĘ£¬ŅŌĢģČ»ĘųĪŖŌĮĻŅ²æÉŅŌÖʵĆCOŗĶH2£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________”£
£Ø2£©ŗĻ³É¼×“¼µÄŅ»ÖÖ·½·ØŹĒŅŌCOŗĶH2ĪŖŌĮĻ£¬ĘäÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£ŗ
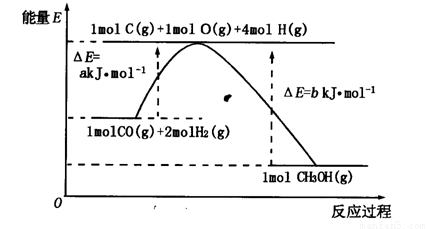
ÓÉĶ¼æÉÖŖ£¬ŗĻ³É¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ________________________________________”£
£Ø3£©ŅŌCO2ĪŖŌĮĻŅ²æÉŅŌŗĻ³É¼×“¼£¬Ęä·“Ó¦ŌĄķĪŖ£ŗCO2(g)+3H2(g)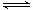 CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
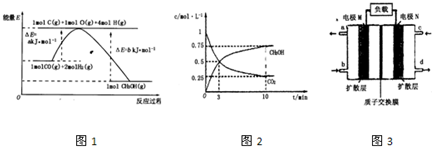
¢ŁŌŚlLµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1molCO2ŗĶ3molH2£¬ŌŚ500”ęĻĀ·¢Éś·“Ó¦£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱĪŹ±ä»ÆČēĶ¼ĖłŹ¾£ŗ
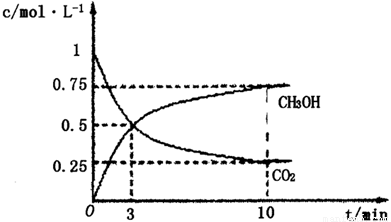
ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ_________________(Ģī×ÖÄø)£»
A£®3minŹ±·“Ó¦“ļµ½Ę½ŗā
B£®0”«10minŹ±ÓĆH2±ķŹ¾µÄ·“Ó¦ĖŁĀŹĪŖ0£®225mol”¤-1”¤min-1
C£®CO2µÄĘ½ŗā×Ŗ»ÆĀŹĪŖ25£„
D£®øĆĪĀ¶ČŹ±»ÆŃ§Ę½ŗā³£ŹżĪŖ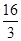 £Ømol/L£©£2
£Ømol/L£©£2
¢ŚŌŚĻąĶ¬ĪĀ¶Č”¢ĻąĶ¬ČŻ»żµÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“²»Ķ¬·½Ź½Ķ¶Čė·“Ó¦Īļ£¬±£³ÖŗćĪĀ”¢ŗćČŻ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ£ŗ
|
ČŻĘ÷ |
ČŻĘ÷1 |
ČŻĘ÷2 |
ČŻĘ÷3 |
|
·“Ó¦ĪļĶ¶ČėĮæ£ØŹ¼Ģ¬£© |
1molCO2Ӣ3molH2 |
0.5molCO2Ӣ1.5molH2 |
1molCH3OHӢ1molH2O |
|
CH3OHµÄĘ½ŗāÅضČ/mol•L-1 |
c1 |
c2 |
c3 |
|
Ę½ŗāŹ±ĢåĻµŃ¹Ēæ/Pa |
p1 |
p2 |
p3 |
ŌņĻĀĮŠø÷ĮæµÄ“󊔹ŲĻµĪŖc1___________c3£¬p2_________p3(Ģī”°“óÓŚ”±”¢”°µČÓŚ”±»ņ”°Š”ÓŚ”±)”£
£Ø4£©½üÄźĄ“£¬¼×“¼Č¼ĮĻµē³Ų¼¼Źõ»ńµĆĮĖŠĀµÄĶ»ĘĘ£¬ČēĶ¼ĖłŹ¾ĪŖ¼×“¼Č¼ĮĻµē³ŲµÄ×°ÖĆŹ¾ŅāĶ¼”£µē³Ų¹¤×÷Ź±£¬·Ö±š“Ób”¢c³äČėCH3OH”¢O2£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
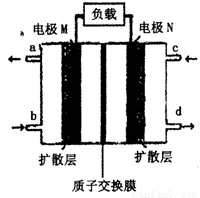
¢Ł“Ód“¦ÅųöµÄĪļÖŹŹĒ___________£¬ČÜŅŗÖŠµÄÖŹ×ÓŅĘĻņµē¼«__________(Ģī”°M”±»ņ”°N”±)£»
¢Śµē¼«MÉĻ·¢ÉśµÄµē¼«·“Ó¦Ź½ĪŖ__________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¹ć¶«Ź”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»Æѧ¾ķ ĢāŠĶ£ŗĢīæÕĢā
¶ŌÓŚČõĖį£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ“ļµ½µēĄėĘ½ŗāŹ±£¬ø÷Ī¢Į£µÄÅØ¶Č“ęŌŚŅ»ÖÖ¶ØĮæµÄ¹ŲĻµ£®ĻĀ±ķŹĒ25”ꏱ¼øÖÖ³£¼ūČõĖįµÄµēĄėĘ½ŗā³£Źż
|
Ėį |
µēĄė·½³ĢŹ½ |
µēĄėĘ½ŗā³£ŹżK |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
»Ų“šĻĀĮŠø÷ĪŹ£ŗ
£Ø1£©KÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬µ±ĪĀ¶ČÉżøߏ±£¬KÖµ________£ØĢī”°Ōö“ó”±”¢”°¼õŠ””±”¢”°²»±ä”±£©£®
£Ø2£©ŌŚĪĀ¶ČĻąĶ¬Ź±£¬ø÷ČõĖįµÄKÖµ²»Ķ¬£¬ÄĒĆ“KÖµµÄ“óŠ”ÓėĖįŠŌµÄĻą¶ŌĒæČõÓŠŗĪ¹ŲĻµ?__________________£®
£Ø3£©Čō°ŃCH3COOH”¢H2CO3”¢HCO3-”¢H2S”¢HS-”¢H3PO4”¢H2PO4-”¢HPO42-¶¼æ“×÷ŹĒĖį£¬ĘäÖŠĖįŠŌ×īĒæµÄŹĒ_________£¬×īČõµÄŹĒ________£®
£Ø4£©¶ąŌŖČõĖįŹĒ·Ö²½µēĄėµÄ£¬ĆæŅ»²½¶¼ÓŠĻąÓ¦µÄµēĄėĘ½ŗā³£Źż£®¶ŌÓŚĶ¬Ņ»ÖÖ¶ąŌŖČõĖįµÄK1”¢K2”¢K3Ö®¼ä“ęŌŚ×ÅŹżĮæÉĻµÄ¹ęĀÉ£¬¶ŌÓŚH3PO4“Ė¹ęĀÉŹĒ________________£¬²śÉś“Ė¹ęĀɵÄŌŅņŹĒ_________________________£®
 £Ø5£©µēĄėĘ½ŗā³£ŹżŹĒÓĆŹµŃéµÄ·½·Ø²ā¶Ø³öĄ“µÄ£®ĻÖŅŃ¾²āµĆijĪĀ¶ČĻĀ NH3∙H2OČÜŅŗÖŠ“ęŌŚČēĻĀ·“Ó¦£ŗNH3∙H2O NH4++OH-
ŅŃÖŖ0.10 mol”¤L-1
NH3∙H2OČÜŅŗÖŠ£¬“ļµ½Ę½ŗāŹ±£¬CĘ½ŗā£ØOH-£©=4.2 ”Į 10-3mol”¤L-1£¬CĘ½ŗā£ØNH3∙H2O£©”ÖCĘšŹ¼£ØNH3∙H2O£©£¬Ė®µÄµēĄėæÉŗöĀŌ²»¼Ę;
£Ø5£©µēĄėĘ½ŗā³£ŹżŹĒÓĆŹµŃéµÄ·½·Ø²ā¶Ø³öĄ“µÄ£®ĻÖŅŃ¾²āµĆijĪĀ¶ČĻĀ NH3∙H2OČÜŅŗÖŠ“ęŌŚČēĻĀ·“Ó¦£ŗNH3∙H2O NH4++OH-
ŅŃÖŖ0.10 mol”¤L-1
NH3∙H2OČÜŅŗÖŠ£¬“ļµ½Ę½ŗāŹ±£¬CĘ½ŗā£ØOH-£©=4.2 ”Į 10-3mol”¤L-1£¬CĘ½ŗā£ØNH3∙H2O£©”ÖCĘšŹ¼£ØNH3∙H2O£©£¬Ė®µÄµēĄėæÉŗöĀŌ²»¼Ę;
¢ŁÓĆpHŹŌÖ½²āĮæČÜŅŗµÄpHÖµ£¬¼“æÉĒóµĆCĘ½ŗā£ØOH-£©£¬²ā¶ØČÜŅŗpHÖµµÄ²Ł×÷ŹĒ______________”£
¢Ś²āĮæCĘ½ŗā£ØNH3∙H2O£©µÄ·½·Ø×īŗĆÓĆ_____________·Ø£ØĢī·½·ØĆū³Ę£©
¢ŪĒó“ĖĪĀ¶ČĻĀøĆ·“Ó¦µÄĘ½ŗā³£ŹżK.(Š“³ö¼ĘĖć¹ż³Ģ£¬¼ĘĖć½į¹ū±£Įō2Ī»ÓŠŠ§Źż×Ö)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com