
”¾ĢāÄæ”湤ŅµÉĻŗĻ³É°±·“Ó¦µÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ
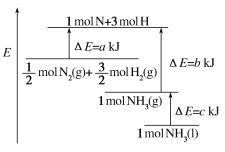
A. N2(g)£«3H2(g)=2NH3(l) ¦¤H£½2(a£b£c)kJ”¤mol£1
B. N2(g)£«3H2(g)=2NH3(g) ¦¤H£½2(b£a)kJ”¤mol£1
C. ![]() N2(g)£«
N2(g)£«![]() H2(g)=NH3(l) ¦¤H£½(b£«c£a)kJ”¤mol£1
H2(g)=NH3(l) ¦¤H£½(b£«c£a)kJ”¤mol£1
D. ![]() N2(g)£«
N2(g)£«![]() H2(g)=NH3(g) ¦¤H£½(a£«b)kJ”¤mol£1
H2(g)=NH3(g) ¦¤H£½(a£«b)kJ”¤mol£1
”¾“š°ø”æA
”¾½āĪö”æ
øł¾Ż·“Ó¦ČȵČÓŚ·“Ó¦Īļ×ÜÄÜĮæ¼õČ„Éś³ÉĪļ×ÜÄÜĮæ¼ĘĖć·“Ó¦ČČ²¢ŹéŠ“ČČ»Æѧ·½³ĢŹ½£¬×¢Ņā·“Ó¦ĪļµÄĪļÖŹµÄĮæŗĶÉś³ÉĪļµÄ¾Ū¼ÆדĢ¬”£
ÓÉĶ¼æÉŅŌ擳ö£¬![]() molN2(g)+
molN2(g)+![]() molH2(g)µÄÄÜĮæĪŖakJ£¬1molNH3(g)µÄÄÜĮæĪŖbkJ£¬ĖłŅŌ
molH2(g)µÄÄÜĮæĪŖakJ£¬1molNH3(g)µÄÄÜĮæĪŖbkJ£¬ĖłŅŌ![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g)£»”÷H=(a-b)kJ/mol£»
H2(g)=NH3(g)£»”÷H=(a-b)kJ/mol£»
¶ų1molµÄNH3(g)×Ŗ»ÆĪŖ1molµÄNH3(l)·Å³öµÄČČĮæĪŖckJ£¬ĖłŅŌÓŠ£ŗ![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l)£»”÷H=(a-b-c)kJ/mol£¬¼“£ŗN2(g)+3H2(g)=2NH3(1)£»”÷H=2(a-b-c)kJmol-1£»¹Ź“š°øĪŖA”£
H2(g)=NH3(l)£»”÷H=(a-b-c)kJ/mol£¬¼“£ŗN2(g)+3H2(g)=2NH3(1)£»”÷H=2(a-b-c)kJmol-1£»¹Ź“š°øĪŖA”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŃõ»Æ»¹ŌŹĒŅ»ĄąÖŲŅŖµÄ·“Ó¦”£
£Ø1£©Ä³Ķ¬Ń§Š“³öŅŌĻĀČżøö»Æѧ·½³ĢŹ½(Ī“ÅäĘ½)
¢ŁN2O4£«H2O”śHNO3£«HNO2 ¢ŚNO£«HNO3”śN2O3£«H2O ¢ŪNH3£«NO”śHNO2£«H2O
ĘäÖŠÄćČĻĪŖŅ»¶Ø²»æÉÄÜŹµĻֵďĒ£ØĢī“śŗÅ£©____________£»
£Ø2£©ŅŌĻĀ·“Ó¦ÖŠH2O2½öĢåĻÖ»¹ŌŠŌµÄŹĒ£ØĢī“śŗÅ£©____________£¬H2O2¼ČĢåĻÖŃõ»ÆŠŌÓÖĢåĻÖ»¹ŌŠŌµÄŹĒ£ØĢī“śŗÅ£©____________£¬“Ó·“Ó¦ÖŠÅŠ¶ĻH2O2”¢Ag2O”¢K2CrO4Ńõ»ÆŠŌÓÉĒæµ½ČõµÄĖ³ŠņŹĒ_______________£»
A£®H2O2+2Fe2++2H+=2Fe3++2H2O
B£®2H2O2=2H2O+O2”ü
C£®Ag2O+H2O2=2Ag+O2”ü+H2O
D£®3H2O2+Cr2(SO4)3+10KOH=2K2CrO4+3K2SO4+8H2O
E£®H2O2+MnSO4=MnO2+H2SO4
£Ø3£©ÓĆ”°Ė«ĻßĒÅ·Ø”±±źĆ÷ŅŌĻĀ·“Ó¦ÖŠµē×Ó×ŖŅʵķ½ĻņŗĶŹżÄæ________£¬Čō·“Ó¦ÖŠÓŠ3.01”Į1023øöµē×Ó×ŖŅĘ£¬Ōņ±»Ńõ»ÆµÄ»¹Ō¼ĮµÄĪļÖŹµÄĮæĪŖ___________”£
2KMnO4£«16HCl£ØÅØ£©=2KCl£«2MnCl2£«5Cl2”ü£«8H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”湤ŅµÉĻÖʱø“æ¹č·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗSiCl4(g)+2H2(g)![]() Si(s)+4HCl(g) ¦¤¦§=+QkJ/mol(Q>0)£¬Ä³ĪĀ¶Č”¢Ń¹ĒæĻĀ£¬½«Ņ»¶ØĮæ·“Ó¦ĪļĶØČėĆܱÕČŻĘ÷½ųŠŠŅŌÉĻ·“Ó¦£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
Si(s)+4HCl(g) ¦¤¦§=+QkJ/mol(Q>0)£¬Ä³ĪĀ¶Č”¢Ń¹ĒæĻĀ£¬½«Ņ»¶ØĮæ·“Ó¦ĪļĶØČėĆܱÕČŻĘ÷½ųŠŠŅŌÉĻ·“Ó¦£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
A.·“Ó¦¹ż³ĢÖŠ£¬ČōŌö“óŃ¹ĒæÄÜĢįøßSiCl4µÄ×Ŗ»ÆĀŹ
B.Čō·“Ó¦æŖŹ¼Ź±SiCl4ĪŖ1mol£¬Ōņ“ļĘ½ŗāŹ±£¬ĪüŹÕČČĮæĪŖQkJ
C.·“Ó¦ÖĮ2minŹ±£¬ČōHClÅضČĪŖ0.12mol/L£¬ŌņH2µÄ·“Ó¦ĖŁĀŹ0.03mol/(L”¤min)
D.µ±·“Ó¦ĪüŹÕČČĮæ0.25QkJŹ±£¬Éś³ÉµÄHClĶØČėŗ¬0.1mol NaOHµÄČÜŅŗĒ”ŗĆ·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijĶ¬Ń§²ÉÓĆ·ĻĢśŠ¼(Ö÷ŅŖ³É·ÖĪŖFe2O3”¢Fe£¬ÉŁĮæĢ¼)ÖĘČ”Ģ¼ĖįŃĒĢś£ØFeCO3£©£¬Éč¼ĘĮĖČēĶ¼Į÷³Ģ£¬øł¾ŻĮ÷³ĢĶ¼£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
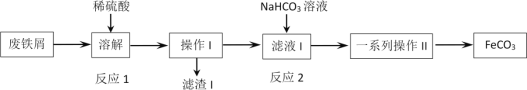
A.¹¤Ņµ·ĻĢśŠ¼ĶłĶłø½×ÅÓŠÓĶÖ¬£¬æÉĶعżČȱ„ŗĶĢ¼ĖįÄĘČÜŅŗĻ“µÓ³żČ„
B.·“Ó¦2µÄĄė×Ó·½³ĢŹ½£ŗFe2++HCO3-=FeCO3”ż+H+
C.²Ł×÷IĪŖ¹żĀĖ£¬Ļ“µÓ²Ł×÷£¬Ņ»ĻµĮŠ²Ł×÷IIĪŖÕō·¢ÅØĖõ£¬ĄäČ“½į¾§£¬¹żĀĖ£¬Ļ“µÓ
D.ĪŖ±ÜĆāĮņĖįČܽāŹ±Fe2+±»æÕĘųŃõ»Æ£¬·ĻĢśŠ¼Ó¦ŹŹµ±¹żĮæ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijŹµŃ銔×éÓĆ100mL0.50mol/LNaOHČÜŅŗÓė60mL0.50mol/LĮņĖį½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£×°ÖĆČēĶ¼ĖłŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
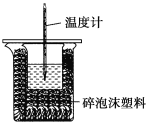
£Ø1£©ČōŹµŃé¹²ŠčŅŖ400mLNaOHČÜŅŗ£¬ŹµŃéŹŅŌŚÅäÖĘøĆČÜŅŗŹ±£¬ŌņŠčŅŖ³ĘĮæNaOH¹ĢĢå____g”£
£Ø2£©Ķ¼ÖŠ×°ÖĆȱɣµÄŅĒĘ÷ŹĒ____”£
£Ø3£©ĮņĖįÉŌ¹żĮæµÄŌŅņŹĒ____”£
£Ø4£©ĒėĢīŠ“ĻĀ±ķÖŠµÄĘ½¾łĪĀ¶Č²ī£ŗ
ŹµŃé “ĪŹż | ĘšŹ¼ĪĀ¶ČT1/”ę | ÖÕÖ¹ĪĀ¶Č T2/”ę | Ę½¾łĪĀ¶Č²ī (T2£T1)/”ę | ||
HCl | NaOH | Ę½¾łÖµ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ____ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
£Ø5£©½üĖĘČĻĪŖ0.50 mol/L NaOHČÜŅŗÓė0.50 mol/LĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻĪŖc=4.18J/(g”ę)ŌņÉĻŹöŹµŃéÖŠŗĶČȦ¤H=___£ØČ”Š”ŹżµćŗóŅ»Ī»£©
£Ø6£©ÉĻŹöŹµŃé½į¹ūÓė57.3kJ/molÓŠĘ«²ī²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ____
A£®ĮæČ”NaOHČÜŅŗŹ±ŃöŹÓ¶ĮŹż
B£®ĪŖĮĖŹ¹·“Ó¦³ä·Ö£¬æÉŅŌĻņĖįÖŠ·Ö“Ī¼ÓČė¼ī
C£®ŹµŃé×°ÖƱ£ĪĀøōČČŠ§¹ū²ī
D£®ÓĆĶĖæ“śĢę²£Į§°ō½Į°č
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¢ń.ŅŃÖŖC”¢H2”¢COµÄČ¼ÉÕČȵďż¾ŻČē±ķĖłŹ¾£ŗ
ĪļÖŹ | C | H2 | CO |
¦¤H/kJ”¤mol£1 | £393.5 | £285.8 | £283.0 |
£Ø1£©Š“³öCĶźČ«Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½£ŗ_____”£
£Ø2£©ÄܱķŹ¾H2Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ____”£
£Ø3£©ĻÖŅŌH2»ņCOĪŖČ¼ĮĻĄ“Ģį¹©ČČÄÜ£¬“ÓČČÄܵĽĒ¶Čæ¼ĀĒ£¬ÄćČĻĪŖ×īŗĆŃ”Ōń__(ĢīŠ“ŠņŗÅ)”£
A£®H2 B£®CO C£®¾łæÉŅŌ
ĄķÓÉŹĒ___”£
¢ņ£®ŅŃÖŖĻĀĮŠČČ»Æѧ·½³ĢŹ½£ŗ
¢ŁH2O(l)=H2(g)£«![]() O2(g) ¦¤H£½£«285.8 kJ/mol
O2(g) ¦¤H£½£«285.8 kJ/mol
¢ŚH2(g)£«![]() O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol
O2(g)=H2O(g) ¦¤H£½£241.8 kJ/mol
¢ŪNaOH(aq)£«HCl(aq)=NaCl(aq)£«H2O(l) ¦¤H£½£57.3 kJ/mol
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø4£©ÉĻŹö·“Ó¦ÖŠŹōÓŚĪüČČ·“Ó¦µÄŹĒ___(ĢīŠņŗÅ)”£
£Ø5£©Č¼ÉÕ10gH2Éś³ÉŅŗĢ¬Ė®£¬·Å³öµÄČČĮæĪŖ___”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A. ³£ĪĀ³£Ń¹ĻĀ£¬8gO2ŗĶO3µÄ»ģŗĻĘųĢåŗ¬ÓŠ4NAøöµē×Ó
B. 1L0.1mol/LµÄFeCl3ĶźČ«Ė®½ā£¬ŠĪ³É0.1NAøöFe(OH)3½ŗĢåĮ£×Ó
C. ±ź×¼×“æöĻĀ£¬22.4LCl2ĶØČėĖ®ÖŠ·¢Éś·“Ó¦£¬×ŖŅʵĵē×ÓŹżĪŖNA
D. 1molNa±»ĶźČ«Ńõ»ÆÉś³ÉNa2O2£¬Ź§Č„øö2NAµē×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹµŃéŹŅÓĆČēĻĀ×°ÖĆÖĘČ”ĀČĘų£¬²¢ÓĆĀČĘų½ųŠŠŹµŃ锣»Ų“šĻĀĮŠĪŹĢā£ŗ
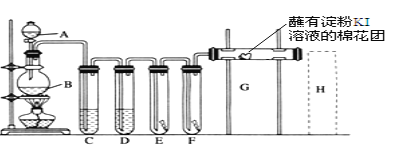
(1)AÖŠŹ¢ÓŠÅØŃĪĖį£¬BÖŠŹ¢ÓŠMnO2£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½___________”£
(2)DÖŠ·ÅČėÅØH2SO4£¬ĘäÄæµÄŹĒ________”£
(3)EÖŠĪŖŗģÉ«øɲ¼Ģõ£¬FÖŠĪŖŗģÉ«ŹŖ²¼Ģõ£¬æɹŪ²ģµ½µÄĻÖĻóŹĒ________£¬¶Ō±ČEŗĶFÖŠĻÖĻóµÄ²īŅģæÉµĆ³öµÄ½įĀŪ¼°½āŹĶ____________________________”£
(4)GŹĒ½žÓŠµķ·ŪKIČÜŅŗµÄĆŽ»ØĒņ£¬G“¦ĻÖĻóŹĒĆŽ»ØĒņ±ķĆę±ä³É______£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ______________£¬HŹĒ½žÓŠNaBrČÜŅŗµÄĆŽ»ØĒņ£¬H“¦ĻÖĻóŹĒĆŽ»ØĒņ±ķĆę±ä³É______”£
(5)»³öH“¦Ī²ĘųĪüŹÕ×°ÖĆĶ¼²¢×¢Ć÷ŹŌ¼Į________”£
(6)ĻÖŌŚÓŠŅ»ÖÖĆūĪŖ”°¾»Ė®Ķč”±µÄ²śĘ·Ņ²ÄܶŌŅūÓĆĖ®½ųŠŠæģĖŁµÄɱ¾śĻū¶¾£¬Ņ©ĶčĶس£·ÖÄŚĶāĮ½²ć”£Ķā²ćµÄÓÅĀČ·Cl2Na(NCO)3ĻČÓėĖ®·“Ó¦£¬Éś³É“ĪĀČĖįʚɱ¾śĻū¶¾×÷ÓĆ£»¼ø·ÖÖÓŗó£¬ÄŚ²ćµÄŃĒĮņĖįÄĘ(Na2SO3)Čܳö£¬æɽ«Ė®ÖŠµÄÓąĀČ(“ĪĀČĖįµČ)³żČ„”£ŃĒĮņĖįÄĘ½«Ė®ÖŠ¶ąÓą“ĪĀČĖį³żČ„µÄĄė×Ó·“Ó¦·½³ĢŹ½ĪŖ________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ŹĒÓŠ¹ŲÄĘÓėĖ®·“Ó¦¼°²śĪļ¼ģŃéµÄŹµŃé×°ÖĆ”£ŹµŃéæŖŹ¼Ź±ĻČĻņ¹ÜÄŚ¼ÓČėµĪÓŠ·ÓĢŖŹŌŅŗµÄĖ®£¬Ź¹Ė®Ī»“ļµ½B¶Ė¹ÜæŚ£¬Č»ŗóŃøĖŁČū½ōĻš½ŗČū²¢°Īµō“óĶ·Õė£¬“ĖŹ±NaµōČėĖ®ÖŠ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
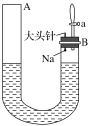
(1)ÄĘÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________”£
(2)ÄĘÓėĖ®·“Ó¦µÄĻÖĻóÓŠŗܶą£¬²»Ķ¬µÄĻÖĻóÖ¤Ć÷²»Ķ¬µÄŠŌÖŹ”£
¢ŁÄÜÖ¤Ć÷ÄʵÄĆܶȱČĖ®Š”µÄĻÖĻóŹĒ_________”£
¢ŚÄÜÖ¤Ć÷ÄʵÄČŪµćµĶµÄĻÖĻóŹĒ_________”£
¢ŪÄÜÖ¤Ć÷ÓŠĒāŃõ»ÆÄĘÉś³ÉµÄĻÖĻóŹĒ__________”£
¢ÜÄÜÖ¤Ć÷ÓŠĘųĢå²śÉśµÄĻÖĻóŹĒA¶ĖŅŗĆę________(Ģī”°ÉĻÉż”±”°ĻĀ½µ”±»ņ”°²»±ä”±£¬ĻĀĶ¬)£¬B¶ĖŅŗĆę________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com