
ij����ˮ�к�5.00��10-3mol��L-1��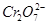 ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ��� ��
�� �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�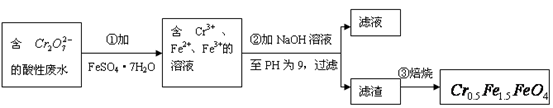
��1���ڢٲ���Ӧ�����ӷ���ʽ��
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
��4����ʹ1L�÷�ˮ�е�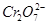 ��ȫת��Ϊ
��ȫת��Ϊ ����������Ҫ����
����������Ҫ����
GFeSO4��7H2O��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)
![]() ij����ˮ�к�5.00��10-3mol?L-1��
ij����ˮ�к�5.00��10-3mol?L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
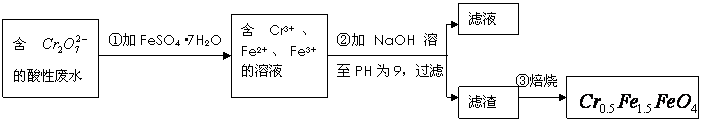
![]()
![]()
![]()
![]()
��1���ڢٲ���Ӧ�����ӷ���ʽ��
![]() ��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
![]()
![]() ��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
![]() ��4����ʹ1L�÷�ˮ�е�
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ���� GFeSO4?7H2O��
����������Ҫ���� GFeSO4?7H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����ˮ�к�5.00��10-3mol��L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�

��1���ڢٲ���Ӧ�����ӷ���ʽ��
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ����ǣ�
��3���ڢڲ����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ����
����������Ҫ����
GFeSO4��7H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
ij����ˮ�к�5.00��10-3mol��L-1�� ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱�
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱�
��Ϊ��������ˮ�����õ����Բ���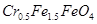 ��
�� �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�


 ��1���ڢٲ���Ӧ�����ӷ���ʽ�� ��
��1���ڢٲ���Ӧ�����ӷ���ʽ�� ��
��2���ڢڲ�����PH��ֽ�ⶨ��ҺPH�IJ��������ǣ�
��
��3���ڢ۲����˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬���� ��
��4����ʹ1L�÷�ˮ�е� ��ȫת��Ϊ
��ȫת��Ϊ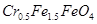 ����������Ҫ����
����������Ҫ����
��FeSO4��7H2O��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com