
�к��ȵIJⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�������ʵ���Ũ�ȵ���Һ��������Ҫ�����������ȣ������ȵĹ�����Ҫ��������������ɢʧ��Ҫ��Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
��1����ѧ��ѧʵ���е��к��ȵIJⶨ����IJ��������У�_________________���ڴ�С�ձ�֮����������ĭ����ֽ������������________________��
��2����ʵ�鳣��0.50 mol?L��1 HCl��0.55 mol?L��1��NaOH��Һ��50 mL��NaOH��Ũ�ȴ���HCl��Ũ��������___________�������µ���10��ʱ���У���ʵ��������ɽϴ�������ԭ����___________��
��3����֪�кͷ�Ӧǰ����Һ���¶ȷֱ�Ϊt1��t2��������HCl��NaOH��Һ���ܶȶ�����Ϊ1 g/cm3���кͺ����ɵ���Һ�ı�����C=4.18 J/��g?�棩������кͷ�Ӧ�ų�����Ϊ______________kJ�������ʽ������H=___________ kJ/mol�������ʽ����
��4��1.00 L 1.00 mol?L��1 H2SO4��Һ��2.00 L 1.00 mol?L��1 NaOH��Һ��ȫ��Ӧ���ų�114.6kJ���������÷�Ӧ���к���Ϊ____ ___����ʾ���к��ȵ��Ȼ�ѧ����ʽΪ
_______ _��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣��к��ȵIJⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�������ʵ���Ũ�ȵ���Һ����ʵ�������Ҫ��������������ɢʧ��Ҫ��Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2���ڴ�С�ձ�֮����������ĭ����ֽ������������____________________�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)
��3����һ���������к���ʵ�飬�¶ȼ���Ҫʹ��______ �Σ�
��4����ʵ�鳣��0��50 mol��L��1 HCl��0��55 mol��L��1��NaOH��Һ��50 mL��������HCl��NaOH��Һ���ܶȶ�����Ϊ1 g/cm3���кͺ����ɵ���Һ�ı�����C = 4.18 J/��g���棩����Ӧ���¶������ˡ�t, ������1molˮʱ�ķ�Ӧ�Ȧ�H=___________kJ/mol�������ʽ����
��5�������50mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡͭ��һ�и߶�10���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��12�֣��к��ȵIJⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�������ʵ���Ũ�ȵ���Һ��������Ҫ�����������ȣ������ȵĹ�����Ҫ��������������ɢʧ��Ҫ��Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
��1����ѧ��ѧʵ���е��к��ȵIJⶨ����IJ��������У�_________ ___________���ڴ�С�ձ�֮����������ĭ����ֽ������������_____ _______________��
��2����ʵ�����0��60 mol��L��1 HCl��0��65 mol��L��1��NaOH��Һ��50 mL��NaOH��Ũ�ȴ���HCl��Ũ��������___ __�������µ���10��ʱ���У���ʵ��������ɽϴ�������ԭ����_________��
��3��������HCl��NaOH��Һ���ܶȶ�����Ϊ1 g/cm3���кͺ����ɵ���Һ�ı�����C=4��18 J/��g���棩������кͷ�Ӧ�ų�����Ϊ_____________kJ�������ʽ������H="___________" kJ/mol�������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�찲��ʡ�߶�10���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��12�֣��к��ȵIJⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�������ʵ���Ũ�ȵ���Һ��������Ҫ�����������ȣ������ȵĹ�����Ҫ��������������ɢʧ��Ҫ��Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
��1����ѧ��ѧʵ���е��к��ȵIJⶨ����IJ��������У�_________ ___________���ڴ�С�ձ�֮����������ĭ����ֽ������������_____ _______________��
��2����ʵ�����0��60 mol��L��1 HCl��0��65 mol��L��1��NaOH��Һ��50 mL��NaOH��Ũ�ȴ���HCl��Ũ��������___ __�������µ���10��ʱ���У���ʵ��������ɽϴ�������ԭ����_________��
��3��������HCl��NaOH��Һ���ܶȶ�����Ϊ1 g/cm3���кͺ����ɵ���Һ�ı�����C=4��18 J/��g���棩������кͷ�Ӧ�ų�����Ϊ_____________kJ�������ʽ������H=___________ kJ/mol�������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�켪��ʡ��һ��ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��14�֣��к��ȵIJⶨʵ��Ĺؼ���Ҫ�Ƚ�ȷ������һ�������ʵ���Ũ�ȵ���Һ����ʵ�������Ҫ��������������ɢʧ��Ҫ��Ƚ�ȷ�ز�������Ӧǰ����Һ�¶ȵı仯���ش��������⣺
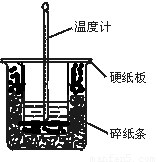
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�� ��
��2���ڴ�С�ձ�֮����������ĭ����ֽ������������____________________�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)
��3����һ���������к���ʵ�飬�¶ȼ���Ҫʹ��______ �Σ�
��4����ʵ�鳣��0��50 mol��L��1 HCl��0��55 mol��L��1��NaOH��Һ��50 mL��������HCl��NaOH��Һ���ܶȶ�����Ϊ1 g/cm3���кͺ����ɵ���Һ�ı�����C = 4.18 J/��g���棩����Ӧ���¶������ˡ�t, ������1molˮʱ�ķ�Ӧ�Ȧ�H=___________ kJ/mol�������ʽ����
��5�������50mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)���������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com