
| A�� | ԭ�����Һ��ֻ����Na+��Fe3+��SO42-�������ܴ���K+��CO32- | |
| B�� | ��ʵ�飨1�����ƶ�ԭ�����Һ���Ƿ���SO42- | |
| C�� | ��ʵ�飨2�����ƶ�ԭ�����Һ���Ƿ���Fe3+ | |
| D�� | ��ʵ�飨3�����ƶ�ԭ�����Һ�д���Fe2+ |
���� ��1����һ�ݼ����������ᣬ���κ����������˵��һ��������CO32-��������Һ�����Կ�֪ԭ��Һ��һ������SO42-��
��2���ڶ��ݼ�������NaOH��Һ�������衢���ˡ�ϴ�ӡ����ա����õ�����ɫ���壬�ú���ɫ�Ĺ���Ϊ��������˵��ԭ��Һ�����ٺ���Fe2+��Fe3+�е�һ�֣�
��3�������ݵμ�0.1mol•L-1����KMnO4��Һ��KMnO4��Һ����ɫ��ʧ��˵��ԭ��Һ�к���Fe2+��
��4�����ýྻ�IJ�˿պȡ�û����Һ����dzɫ���������գ����ֻ�����ֻ�ɫ��˵��ԭ��Һ��һ������Na+���ݴ˽��н��
��� �⣺���ݣ�1����֪ԭ��Һ��һ��������CO32-�������Һ�����Կ�֪ԭ��Һ��һ������SO42-�����ݣ�2����֪���ú���ɫ�Ĺ���Ϊ��������˵��ԭ��Һ�����ٺ���Fe2+��Fe3+�е�һ�֣����ݣ�3��KMnO4��Һ����ɫ��ʧ��˵��ԭ��Һ�к���Fe2+�����ݣ�4��������ֻ�ɫ��˵��ԭ��Һ��һ������Na+��
A��ԭ�����Һ��һ������Na+��Fe2+��SO42-��һ��������CO32-����ȷ���Ƿ���K+����A����
B�����ݣ�1����֪һ��������CO32-�������Һ�����Կ�֪ԭ��Һ��һ������SO42-����B����
C�����ݷ�����֪����ʵ�飨2�����ƶ�ԭ�����Һ���Ƿ���Fe3+����C��ȷ��
D����ʵ�飨3�����ƶ�ԭ�����Һ��һ������Fe2+����D��ȷ��
��ѡCD��
���� ���⿼���˳������ӵļ��鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ�����Ϊ���ؼ���ע���������ճ������ӵļ��鷽��������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢۢߢ��� | B�� | �٢ڢۢݢ�� | C�� | �ڢݢޢߢ� | D�� | �ڢܢߢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
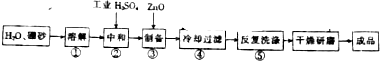
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
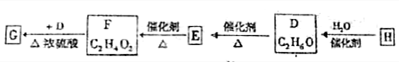

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
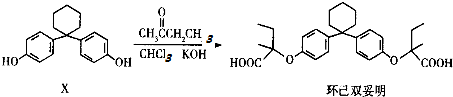
| A�� | �л���X�ķ���ʽΪC18H22O2 | |
| B�� | �л���X�뻷��˫������Ϊͬϵ�� | |
| C�� | ����˫���������ϵ�һ�ȴ�����4�� | |
| D�� | ����˫��������̼��������Һ������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��һ����KAl��SO4��2��Һ��һ����Ba��OH��2��Һ��ϣ�����������ǡ�����ʱ��Al3++2SO42-+3OH-+2Ba2+�T2BaSO4��+Al��OH��3�� | |
| B�� | ��Ba��OH��2��Һ�еμ�NaHSO4��Һ�������Һǡ��Ϊ���ԣ�Ba2++OH-+H++SO42-�TBaSO4��+H2O | |
| C�� | FeCl3��Һ��Cu�ķ�Ӧ��Cu+Fe3+�TCu2++Fe2+ | |
| D�� | ��Al��OH��3�к����θ�Al��OH��3+3H+�TAl3++3H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
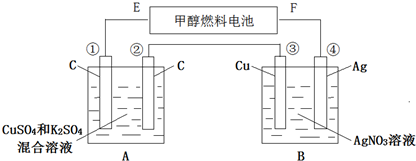
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ĵ缫��ӦʽΪ��O2+4H++4e-�T2H2O | |
| B�� | ��Һ���������������ƶ� | |
| C�� | �����ĵ缫��ӦʽΪ��N2H4+4OH--4e-�T4H2O+N2 | |
| D�� | �������Һ��pH���ֲ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com