
��ҩ��C��������N�Լ��߷�����֬(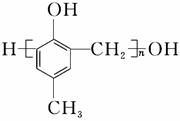 )�ĺϳ�·�����£�
)�ĺϳ�·�����£�
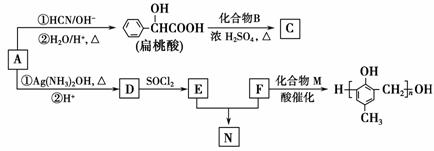
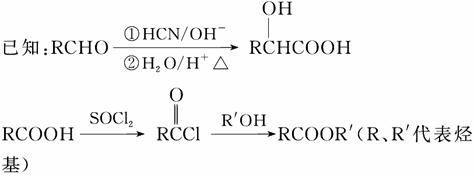
(1)A�ĺ��������ŵ�������________��
(2)A�ڴ��������¿���H2��Ӧ����B���÷�Ӧ�ķ�Ӧ������________��
(3)�������C�ķ���ʽ��C15H14O3����ṹ��ʽ��________________��
(4)A����������Ӧ�Ļ�ѧ����ʽ��______________________________
_____________________________________________________________��
(5)������(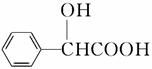 )�ж���ͬ���칹�塣���ڼ������Һ����ǻ���ͬ���칹�干��________�֣�д������һ�ֺ��Ǽ�(��CH2��)��ͬ���칹��Ľṹ��ʽ______________________��
)�ж���ͬ���칹�塣���ڼ������Һ����ǻ���ͬ���칹�干��________�֣�д������һ�ֺ��Ǽ�(��CH2��)��ͬ���칹��Ľṹ��ʽ______________________��
(6)F��M�ϳɸ߷�����֬�Ļ�ѧ����ʽ��___________________________
______________________________________________________________��
(7)N��NaOH��Һ�з���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
___________________________________________________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A�������еμӰ�ˮ�����ԣ���Һ������Ϊ�Ȼ��
B��ϡ�����ˮϡ�ͣ��������̶�������Һ��pH��С
C������ʯ��ˮ�м�������CaO���ָ������º���Һ��pH����
D����ˮ�еμ���������FeCl3��Һ���γɴ���Ľ��壬����������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݵ���������Ӧ���Ȼ�ѧ����ʽ��
(ⅰ)I2(g)��H2(g)2HI(g)
��H����9.48 kJ·mol��1
(ⅱ)I2(s)��H2(g)2HI(g)
��H����26.48 kJ·mol��1
�����ж���ȷ����(����)
A��254 g I2(g)��ͨ��2 g H2(g)����Ӧ����9.48 kJ
B��1 mol��̬����1 mol��̬���������������17.00 kJ
C����Ӧ(ⅰ)�IJ���ȷ�Ӧ(ⅱ)�IJ����ȶ�
D����Ӧ(ⅱ)�ķ�Ӧ���������ȷ�Ӧ(ⅰ)�ķ�Ӧ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������糧�ͷų������ĵ�������(NOx)����������Ͷ�����̼���������ɻ�����Ⱦ����ȼ�շ���������������̼������ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ġ�
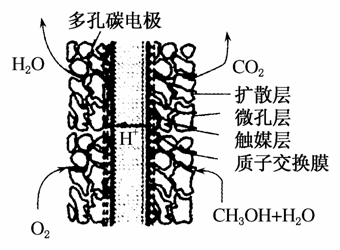
(1)���������ü������ԭNO2��
CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)
��H����574 kJ·mol��1
CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g)
��H����1 160 kJ·mol��1
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ________________
_________________________________________________________��
(2)ijһ�״�ȼ�ϵ�صĽṹ��ͼ��ʾ���乤��ʱ�����ĵ缫��Ӧʽ�ɱ�ʾΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ��Һ���ǿ�ѧ�ҽ������Ƴ���һ��ʹɳĮ�������¼�������������ɳĮ������һ�����ľ۱�ϩ����(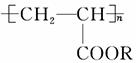 )��ˮ��Һ������ˮ��Һ���еĸ߷��ӻ�������ɳ���������ϣ��ڵر���30��50 cm���γ�һ����0.5 cm�ĸ�ˮ�㣬������ֹ���µ��η��������������������ˮ�����á����й��ھ۱�ϩ������˵���У�����ȷ���� (����)��
)��ˮ��Һ������ˮ��Һ���еĸ߷��ӻ�������ɳ���������ϣ��ڵر���30��50 cm���γ�һ����0.5 cm�ĸ�ˮ�㣬������ֹ���µ��η��������������������ˮ�����á����й��ھ۱�ϩ������˵���У�����ȷ���� (����)��
A���ϳ�����С���ӻ�������CH3��CH2��COOR
B��������CH2==CH��COOR�����ӳɾۺϷ�Ӧ���õ�
C����һ���������ܷ���ˮ�ⷴӦ
D�����ܷ����ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС���Ա�����ϩΪ��Ҫԭ�ϣ���������·�ߺϳ�ҩ����³����


��ش��������⣺
(1)������³��������˵����ȷ����________��
A������Ũ�����γ���
B���������������ӳɷ�Ӧ
C���ɷ���ˮ�ⷴӦ
D���������
(2)д��������B�Ľṹ��ʽ________________��
(3)д��B��C��Ӧ������Լ�________��
(4)д��C��D�D��E�Ļ�ѧ��Ӧ����ʽ_____________________________
_______________________________________________________________��
(5)д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ________��
�ٷ����к����Ȼ�
��1HNMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ��
(6)ͨ��������ϩΪԭ���Ƶû����������X��Ӧ�ϳ�D�����û�ѧ��Ӧ����ʽ��ʾ����ϩΪԭ���Ʊ�X�ĺϳ�·��(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.48 g Mg�ۼ��뵽500 mL 0.1 mol��L��1��������ǡ����ȫ��Ӧ����ԭ��������� (����)��
A��NO2 B��NO
C��N2O3 D��NH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ں˵����1��18��Ԫ���У��䵥�����ڽ����������____________________�������У��ܶ���С����________���ؿ��к������Ľ���Ԫ����________���۵���͵���________���������ᷴӦ���ܼӦ����________�����ʵĻ�ԭ����ǿ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ���κ��¶��¾����Է����е��� (����)��
A��2N2(g)��O2(g)===2N2O(g) ����H����163 kJ��mol��1
B��Ag(s)��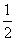 Cl2(g)===AgCl(s) ����H����127 kJ��mol��1
Cl2(g)===AgCl(s) ����H����127 kJ��mol��1
C��HgO(s)===Hg(l)��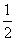 O2(g)����H����91 kJ��mol��1
O2(g)����H����91 kJ��mol��1
D��H2O2(l)===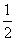 O2(g)��H2O(l)����H����98 kJ��mol��1
O2(g)��H2O(l)����H����98 kJ��mol��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com