
(12��)ʵ������ȡ��ϩ��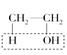 ŨH2SO4170 ��C2H4����H2O�����¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ��������SO2�������������ʵ����ȷ�������������������ϩ��SO2��
ŨH2SO4170 ��C2H4����H2O�����¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ��������SO2�������������ʵ����ȷ�������������������ϩ��SO2��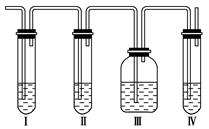
(1)��װ��ʢ�ŵ��Լ��ǣ���______����______����________����________(���й��Լ���������ں�����)��
| A��Ʒ����Һ | B��NaOH��Һ |
| C��ŨH2SO4��Һ | D������KMnO4��Һ |
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ʵ������ȡ��ϩ��ŨH2SO4170 ��C2H4����H2O�����¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ��������SO2�������������ʵ����ȷ�������������������ϩ��SO2��
(1)��װ��ʢ�ŵ��Լ��ǣ���______����______����________����________(���й��Լ���������ں�����)��
A��Ʒ����Һ B��NaOH��Һ
C��ŨH2SO4��Һ D������KMnO4��Һ
(2)��˵��SO2������ڵ�������__________________��
(3)ʹ��װ�â��Ŀ����________________________________________________��
ʹ��װ�â��Ŀ����________________��
(4)ȷ�Ϻ���ϩ��������________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ʦ���и�һ��ѧ����ĩ���⻯ѧ�������Ծ� ���ͣ������
(12��)ʵ������ȡCl2�����ʵ���֤ʵ�����װ�����£�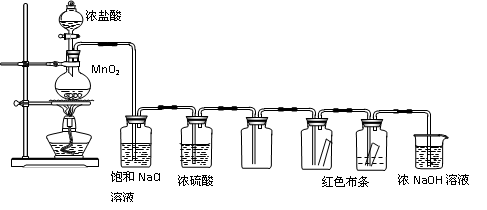
A B C D E F G
�ش��������⣺
(1)A�еĻ�ѧ����ʽΪ ��
(2)B�������dz�ȥHCl���壬C�������� ��
(3)E��F�й۲쵽������ֱ��� ����õ��Ľ����� ��
(4)G�������� ��
��ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�߶������¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(12��)ʵ������ȡ��ϩ��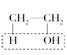 ŨH2SO4170 ��C2H4����H2O�����¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ��������SO2�������������ʵ����ȷ�������������������ϩ��SO2��
ŨH2SO4170 ��C2H4����H2O�����¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ��������SO2�������������ʵ����ȷ�������������������ϩ��SO2��
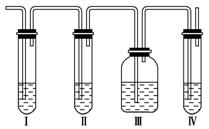
(1)��װ��ʢ�ŵ��Լ��ǣ���______����______����________����________(���й��Լ���������ں�����)��
A��Ʒ����Һ B��NaOH��Һ
C��ŨH2SO4��Һ D������KMnO4��Һ
(2)��˵��SO2������ڵ�������__________________��
(3)ʹ��װ�â��Ŀ����________________________________________________��
ʹ��װ�â��Ŀ����________________��
(4)ȷ�Ϻ���ϩ��������________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ������
(12��) ʵ��������������(��Ҫ�ɷ���Al2O3��������SiO2��Fe2O3����)Ϊԭ����ȡAl2(SO4)3�����������[NH4Al(SO4)2��12H2O]�Ĺ����������£�
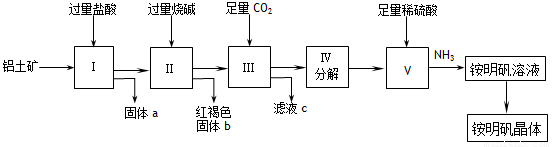
�Իش��������⣺
�Ź���a�Ļ�ѧʽΪ_____��III��ͨ�����CO2��������ӷ���ʽΪ ��
����V��ȡ�������Һ�Ļ�ѧ����ʽΪ__________________________�����������Һ�л������������ʵ���������Ϊ����������ƣ� ����ȴ�ᾧ������ϴ�ӡ�
����1000kg��������36%��������Ϊԭ����ȡAl2(SO4)3����������������98%�����ᣨ�ܶ�1.84g/cm3��___________L������һλС������
����ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1:1����Ͷ��ʱ��������Al2O3��H2SO4�����ʵ���֮��Ϊ___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com