
| A£®Ņ»¶Ø²»“ęŌŚBa2+£¬æÉÄÜ“ęŌŚNH4+ |
| B£®²»æÉÄÜ“ęŌŚBa2+”¢Cl- |
| C£®Na+Ņ»¶Ø“ęŌŚ£¬ĒŅc(Na+)”Ż0.2 mol/L |
| D£®Na+”¢Cl-æÉÄÜ“ęŌŚ |


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĪŽÉ«ČÜŅŗ:K+”¢Al3+”¢NO3”Ŗ”¢AlO2”Ŗ |
| B£®pH=12µÄČÜŅŗ:K+”¢Cl-”¢SO32”Ŗ”¢SiO32”Ŗ |
| C£®ŗ¬0.1 mol”¤L-1 NO3”ŖµÄČÜŅŗ:H+”¢Fe2+”¢Cl-”¢SO42”Ŗ |
| D£®ÓÉĖ®µēĄė²śÉśµÄc(H+)=1”Į10-12 mol”¤L-1µÄČÜŅŗ:Na+”¢NH4+”¢SO42”Ŗ”¢HCO3”Ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Fe2+”¢Mg2+”¢ClO-”¢Cl-ÄÜ“óĮæ¹²“ę |
| B£®ŗ¬Br-”¢K+”¢Na+”¢HSO3-µÄČÜŅŗ£¬ĶØČėSO2ŗóÕāŠ©Ąė×ÓČŌÄÜ“óĮæ¹²“ę |
C£®ŗ¬K+”¢Na+”¢NO3-”¢CH3COO-µÄČÜŅŗÖŠc£ØH+£©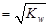 |
| D£®ĻõĖįÄĘŗĶµā»Æ±µ»ģŗĻČÜŅŗÖŠ£¬ČÜÖŹĄė×ÓÅضČĪŖc £ØBa2+£©=0£®2mol/L£¬c£ØNa+£©=0£®2mol/L£¬c£ØNO3-£©=0£®3mol/L£¬c£ØI-£©=0£®1mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Čē¹ūa1b1£½a2b2£¬Ōņ»ģŗĻČÜŅŗµÄpH£½7 |
| B£®Čē¹ū»ģŗĻŅŗµÄpH£½7£¬Ōņ»ģŗĻČÜŅŗÖŠc(CH3COO£)£½c(Na£«) |
| C£®Čē¹ūa1£½a2£¬b1£½b2£¬Ōņ»ģŗĻČÜŅŗÖŠc(CH3COO£)£½c(Na£«) |
| D£®Čē¹ūa1£½a2£¬ĒŅ»ģŗĻČÜŅŗµÄpH>7£¬Ōņb1<b2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ČÜŅŗµÄpH£ŗ¢Ś>¢Ū>¢Ł |
| B£®Ė®µēĄė³öµÄc(OH-)£ŗ¢Ū>¢Ł>¢Ś |
| C£®¢ŚŗĶ¢ŪµČĢå»ż»ģŗĻŗóµÄČÜŅŗ£ŗc(Na+)+c(H+)=c(OH-)+c(CH3COO-) |
| D£®¢ŁŗĶ¢ŚµČĢå»ż»ģŗĻŗóµÄČÜŅŗ£ŗc(CH3COOH)+c(CH3COO-)="0.1" mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¼×»ł³Č³ŹŗģÉ«µÄČÜŅŗÖŠ£ŗMnO4£”¢Al3£«”¢C2H5OH”¢SO42- |
| B£®Ė®µēĄė³öµÄc(H£«)£½1”Į10£14 mol”¤L£1µÄČÜŅŗÖŠ£ŗCa2£«”¢NH4£«”¢Cl£”¢SiO32£ |
| C£®c(H£«)/c(OH£)£½1012µÄČÜŅŗÖŠ£ŗNH4+”¢Al3£«”¢NO3-”¢ClO£ |
| D£®c(NaHCO3)£½0.1 mol”¤L£1µÄČÜŅŗÖŠ£ŗK£«”¢C6H5O£”¢SO42-”¢CO32£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®H+”¢K+”¢Fe2+”¢NO3£ | B£®OH£”¢Cl£”¢Na+”¢NH4+ |
| C£®Mg2+”¢K+”¢Cl£”¢NO3£ | D£®Cu2+”¢NO3£”¢OH£”¢Cl£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®µĪ¼Ó¼×»ł³ČŹŌ¼ĮĻŌŗģÉ«µÄČÜŅŗÖŠ£ŗNa£«”¢Fe2£«”¢Cl£”¢NO3£ |
| B£®[H+]=10-12 mol”¤L-1µÄČÜŅŗ£ŗK+”¢Ba2+”¢Cl-”¢Br- |
| C£®[OH£]/ [H+]£½1012µÄČÜŅŗÖŠ£ŗNH4£«”¢Al3£«”¢NO3£”¢CO32£ |
| D£®ÓÉĖ®µēĄėµÄ[H+]£½1.0”Į10£13 mol”¤L£1µÄČÜŅŗÖŠ£ŗK£«”¢NH4£«”¢[Al(OH)4]£”¢HCO3£ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com