
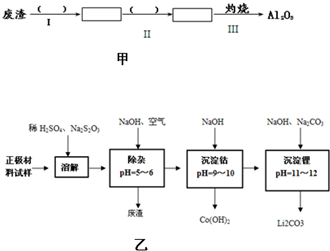
| ³ĮµķĪļ | æŖŹ¼³ĮµķpH | ³ĮµķĶźČ«pH |
| Al£ØOH£©3 | 3.0 | 5.2 |
| Fe£ØOH£©3 | 1.5 | 2.8 |
| Fe£ØOH£©2 | 7.6 | 9.7 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ³ĮµķĪļ | æŖŹ¼³ĮµķpH | ³ĮµķĶźČ«pH |
| Al£ØOH£©3 | 3.0 | 5.2 |
| Fe£ØOH£©3 | 1.5 | 2.8 |
| Fe£ØOH£©2 | 7.6 | 9.7 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
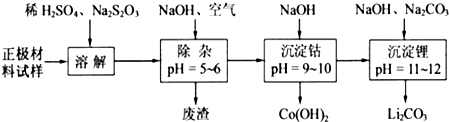
Õż¼«²ÄĮĻĪŖ![]() µÄļ®Ąė×Óµē³ŲŅѱ»¹ć·ŗÓĆ×÷±ćŠÆŹ½µēŌ“”£µ«īܵÄ׏Ō“ŲŃ·¦ĻŽÖĘĮĖĘä½ųŅ»²½·¢Õ¹”£
µÄļ®Ąė×Óµē³ŲŅѱ»¹ć·ŗÓĆ×÷±ćŠÆŹ½µēŌ“”£µ«īܵÄ׏Ō“ŲŃ·¦ĻŽÖĘĮĖĘä½ųŅ»²½·¢Õ¹”£
£Ø1£©éĻéŹÆŠĶ![]() ŹĒŅ»ÖÖĒ±ŌŚµÄļ®Ąė×Óµē³ŲÕż¼«²ÄĮĻ£¬ĖüæÉŅŌĶعż
ŹĒŅ»ÖÖĒ±ŌŚµÄļ®Ąė×Óµē³ŲÕż¼«²ÄĮĻ£¬ĖüæÉŅŌĶعż![]() ”¢
”¢![]() Óė
Óė![]() ČÜŅŗ·¢Éś¹²³Įµķ·“Ó¦£¬ĖłµĆ³Įµķ¾80”ęÕęæÕøÉŌļ”¢øßĪĀ³ÉŠĶ¶ųÖʵƔ£
ČÜŅŗ·¢Éś¹²³Įµķ·“Ó¦£¬ĖłµĆ³Įµķ¾80”ęÕęæÕøÉŌļ”¢øßĪĀ³ÉŠĶ¶ųÖʵƔ£
¢Ł¹²³Įµķ·“Ó¦Ķ¶ĮĻŹ±£¬²»½«![]() ŗĶ
ŗĶ![]() ČÜŅŗÖ±½Ó»ģŗĻµÄŌŅņŹĒ ”£
ČÜŅŗÖ±½Ó»ģŗĻµÄŌŅņŹĒ ”£
¢Ś¹²³Įµķ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢ŪøßĪĀ³ÉŠĶĒ°£¬³£Ļņ![]() ÖŠ¼ÓČėÉŁĮæ»īŠŌĢæŗŚ£¬Ęä×÷ÓĆ³żĮĖæÉŅŌøÄÉĘ³ÉŠĶŗóµÄ
ÖŠ¼ÓČėÉŁĮæ»īŠŌĢæŗŚ£¬Ęä×÷ÓĆ³żĮĖæÉŅŌøÄÉĘ³ÉŠĶŗóµÄ![]() µÄµ¼µēŠŌÄÜĶā£¬»¹ÄÜ ”£
µÄµ¼µēŠŌÄÜĶā£¬»¹ÄÜ ”£
£Ø2£©·Ļ¾Éļ®Ąė×Óµē³ŲµÄÕż¼«²ÄĮĻŹŌŃł£ØÖ÷ŅŖŗ¬ÓŠ![]() ¼°ÉŁĮæAI”¢FeµČ£©æÉĶعżĻĀĮŠŹµŃé·½·Ø»ŲŹÕīÜ”¢ļ®”£
¼°ÉŁĮæAI”¢FeµČ£©æÉĶعżĻĀĮŠŹµŃé·½·Ø»ŲŹÕīÜ”¢ļ®”£

ŌŚÉĻŹöČܽā¹ż³ĢÖŠ£¬![]() ±»Ńõ»Æ³É
±»Ńõ»Æ³É![]() £¬
£¬![]() ŌŚČܽā¹ż³ĢÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
ŌŚČܽā¹ż³ĢÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
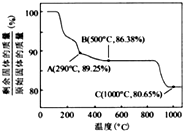
![]() ŌŚæÕĘųÖŠ¼ÓČČŹ±£¬¹ĢĢ岊ĮōĀŹĖęĪĀ¶ČµÄ±ä»Æ£¬ČēĻĀĶ¼ĖłŹ¾”£ŅŃÖŖīܵÄĒāŃõ»ÆĪļ¼ÓČČÖĮ290”ꏱŅŃĶźČ«ĶŃĖ®£¬Ōņ1000”ꏱ£¬Ź£Óą¹ĢĢåµÄ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©£»ŌŚ350~400”ę·¶Ī§ÄŚ£¬Ź£Óą¹ĢĢåµÄ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©”£
ŌŚæÕĘųÖŠ¼ÓČČŹ±£¬¹ĢĢ岊ĮōĀŹĖęĪĀ¶ČµÄ±ä»Æ£¬ČēĻĀĶ¼ĖłŹ¾”£ŅŃÖŖīܵÄĒāŃõ»ÆĪļ¼ÓČČÖĮ290”ꏱŅŃĶźČ«ĶŃĖ®£¬Ōņ1000”ꏱ£¬Ź£Óą¹ĢĢåµÄ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©£»ŌŚ350~400”ę·¶Ī§ÄŚ£¬Ź£Óą¹ĢĢåµÄ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äźøßæ¼ŹŌĢā--»Æѧ£Ø½ĖÕ¾ķ£©½āĪö ĢāŠĶ£ŗŹµŃéĢā
Õż¼«²ÄĮĻĪŖ µÄļ®Ąė×Óµē³ŲŅѱ»¹ć·ŗÓĆ×÷±ćŠÆŹ½µēŌ“”£µ«īܵÄ׏Ō“ŲŃ·¦ĻŽÖĘĮĖĘä½ųŅ»²½·¢Õ¹”£
µÄļ®Ąė×Óµē³ŲŅѱ»¹ć·ŗÓĆ×÷±ćŠÆŹ½µēŌ“”£µ«īܵÄ׏Ō“ŲŃ·¦ĻŽÖĘĮĖĘä½ųŅ»²½·¢Õ¹”£
£Ø1£©éĻéŹÆŠĶ ŹĒŅ»ÖÖĒ±ŌŚµÄļ®Ąė×Óµē³ŲÕż¼«²ÄĮĻ£¬ĖüæÉŅŌĶعż
ŹĒŅ»ÖÖĒ±ŌŚµÄļ®Ąė×Óµē³ŲÕż¼«²ÄĮĻ£¬ĖüæÉŅŌĶعż ”¢
”¢ Óė
Óė ČÜŅŗ·¢Éś¹²³Įµķ·“Ó¦£¬ĖłµĆ³Įµķ¾80”ęÕęæÕøÉŌļ”¢øßĪĀ³ÉŠĶ¶ųÖʵƔ£
ČÜŅŗ·¢Éś¹²³Įµķ·“Ó¦£¬ĖłµĆ³Įµķ¾80”ęÕęæÕøÉŌļ”¢øßĪĀ³ÉŠĶ¶ųÖʵƔ£
¢Ł¹²³Įµķ·“Ó¦Ķ¶ĮĻŹ±£¬²»½« ŗĶ
ŗĶ ČÜŅŗÖ±½Ó»ģŗĻµÄŌŅņŹĒ
ӣ
ČÜŅŗÖ±½Ó»ģŗĻµÄŌŅņŹĒ
ӣ
¢Ś¹²³Įµķ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢ŪøßĪĀ³ÉŠĶĒ°£¬³£Ļņ ÖŠ¼ÓČėÉŁĮæ»īŠŌĢæŗŚ£¬Ęä×÷ÓĆ³żĮĖæÉŅŌøÄÉĘ³ÉŠĶŗóµÄ
ÖŠ¼ÓČėÉŁĮæ»īŠŌĢæŗŚ£¬Ęä×÷ÓĆ³żĮĖæÉŅŌøÄÉĘ³ÉŠĶŗóµÄ µÄµ¼µēŠŌÄÜĶā£¬»¹ÄÜ
ӣ
µÄµ¼µēŠŌÄÜĶā£¬»¹ÄÜ
ӣ
£Ø2£©·Ļ¾Éļ®Ąė×Óµē³ŲµÄÕż¼«²ÄĮĻŹŌŃł£ØÖ÷ŅŖŗ¬ÓŠ ¼°ÉŁĮæAI”¢FeµČ£©æÉĶعżĻĀĮŠŹµŃé·½·Ø»ŲŹÕīÜ”¢ļ®”£
¼°ÉŁĮæAI”¢FeµČ£©æÉĶعżĻĀĮŠŹµŃé·½·Ø»ŲŹÕīÜ”¢ļ®”£

¢Ł  ŌŚÉĻŹöČܽā¹ż³ĢÖŠ£¬
ŌŚÉĻŹöČܽā¹ż³ĢÖŠ£¬ ±»Ńõ»Æ³É
±»Ńõ»Æ³É £¬
£¬ ŌŚČܽā¹ż³ĢÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
ӣ
ŌŚČܽā¹ż³ĢÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
ӣ
¢Ś  ŌŚæÕĘųÖŠ¼ÓČČŹ±£¬¹ĢĢ岊ĮōĀŹĖęĪĀ¶ČµÄ±ä»Æ
ŌŚæÕĘųÖŠ¼ÓČČŹ±£¬¹ĢĢ岊ĮōĀŹĖęĪĀ¶ČµÄ±ä»Æ
ČēÓŅĶ¼ĖłŹ¾”£ŅŃÖŖīܵÄĒāŃõ»ÆĪļ¼ÓČČÖĮ290”ꏱŅŃĶźČ«
ĶŃĖ®£¬Ōņ1000”ꏱ£¬Ź£Óą¹ĢĢåµÄ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©£»
ŌŚ350~400”ę·¶Ī§ÄŚ£¬Ź£Óą¹ĢĢåµÄ³É·ÖĪŖ ”££ØĢī»ÆѧŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com