��2.4mol/L��H
2SO
4��Һ����100mLŨ��Ϊ0.2mol/L��ϡH
2SO
4���ش��������⣺
��1��������Ͳ��ȡ2.4mol/L��H
2SO
4��Һ�������
8.3
8.3
mL��
��2����Һ���Ƶ�����Ļ����������£�
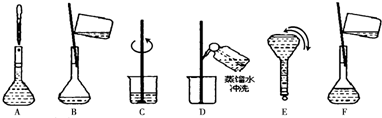
������ʵ�鲽��A��F��ʵ������Ⱥ��������
CBDFAE
CBDFAE
��
��3������ʵ�鲽��A��B��E��F���õ�����������Ϊ
100mL����ƿ
100mL����ƿ
��
��4��ȡ����������Һ10mL������BaCl
2��Һ��Ӧ�����ɰ�ɫ����0.48g�������ҺŨ��
����
����
0.2mol/L������ڡ������ڡ���С�ڡ�������ɴ����IJ���������
a
a
��
a������ʱ��������ƿ�� b������Ͳȡ2.4mol/LH
2SO
4��Һʱ���Ӷ�����
c������ƿʹ��ǰδ���d��ʹ�õ��ձ��Ͳ�����δϴ�ӳ��ף�e������ʱ������ˮ��������ƿ���森