
±ūĻ©ĖįŅŅõ„£Ø»ÆŗĻĪļ¢ō£©ŹĒÖʱøĖÜĮĻ”¢Ź÷Ö¬µČøß¾ŪĪļµÄÖŲŅŖÖŠ¼äĢ壬æÉÓÉĻĀĆęĀ·ĻßŗĻ³É£ŗ
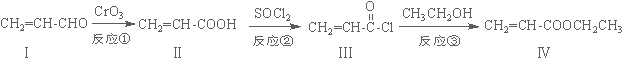 £Ø1£©»ÆŗĻĪļ¢ōµÄ·Ö×ÓŹ½ĪŖ £¬1 mol»ÆŗĻĪļ¢ōĶźČ«Č¼ÉÕĻūŗÄO2ĪŖ mol”£
£Ø1£©»ÆŗĻĪļ¢ōµÄ·Ö×ÓŹ½ĪŖ £¬1 mol»ÆŗĻĪļ¢ōĶźČ«Č¼ÉÕĻūŗÄO2ĪŖ mol”£
£Ø2£©»ÆŗĻĪļ¢ņÄÜŹ¹äåĖ®ĶŹÉ«£¬Ęä·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©·“Ó¦¢ŚŹōÓŚ ·“Ó¦£¬»ÆŗĻĪļ¢ńæÉŅŌÓÉ»ÆŗĻĪļ¢õ£Ø·Ö×ÓŹ½ĪŖC3H6O£©“ß»ÆŃõ»ÆµĆµ½£¬Ōņ»ÆŗĻĪļ¢õ”ś¢ńµÄ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø4£©»ÆŗĻĪļ¢öŹĒ»ÆŗĻĪļ¢ōµÄĶ¬·ÖŅģ¹¹Ģ壬¢öŗ¬ÓŠĢ¼Ģ¼Ė«¼ü²¢ÄÜÓėNaHCO3ČÜŅŗ·“Ó¦·Å³öĘųĢ壬ĘäŗĖ“Ź²ÕńĒāĘ×·åĆ껿֮±ČĪŖ1£ŗ1£ŗ6£¬Ōņ»ÆŗĻĪļ¢öµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø5£©Ņ»¶ØĢõ¼žĻĀ£¬»ÆŗĻĪļ  Ņ²æÉÓė»ÆŗĻĪļ¢ó·¢ÉśĄąĖĘ·“Ó¦¢ŪµÄ·“Ó¦£¬ŌņµĆµ½µÄ²śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
Ņ²æÉÓė»ÆŗĻĪļ¢ó·¢ÉśĄąĖĘ·“Ó¦¢ŪµÄ·“Ó¦£¬ŌņµĆµ½µÄ²śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
”¾ÖŖŹ¶µć”æÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ L2 L4 L7
”¾“š°ø½āĪö”æ(1)C5H8O2 £» 6£»
(2)CH2=CH-COOH+Br2”śCH2Br-CHBr-COOH
(3)Č”“ś 2CH2=CH-CH2OH+O2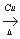 2CH2=CH-CHO+2H2O
2CH2=CH-CHO+2H2O
(4)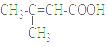 (5)
(5)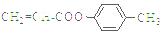
½āĪö£ŗ£Ø1£©»ÆŗĻĪļ¢ōµÄŅ»øö·Ö×ÓÓŠ5øöĢ¼Ō×Ó”¢8øöĒāŌ×Ó”¢2øöŃõŌ×Ó£¬·Ö×ÓŹ½ĪŖC5H8O2£¬1 mol»ÆŗĻĪļ¢ōĶźČ«Č¼ÉÕĻūŗÄO2ĪŖ£ŗ£Ø5+2-1£©=6mol”£
£Ø2£©»ÆŗĻĪļ¢ņµÄ·Ö×Ó½į¹¹ÖŠŗ¬Ģ¼Ģ¼Ė«¼ü£¬ÓėäåĖ®·¢Éś¼Ó³É·“Ó¦£¬Ęä·“Ó¦·½³ĢŹ½£ŗCH2=CH-COOH+Br2”śCH2Br-CHBr-COOH
£Ø3£©»ÆŗĻĪļ¢ņ·Ö×Ó½į¹¹ÖŠµÄōĒ»ł±»ĀČŌ×ÓČ”“ś£»»ÆŗĻĪļ¢ńæÉŅŌÓÉ»ÆŗĻĪļ¢õ£Ø·Ö×ÓŹ½ĪŖC3H6O£©“ß»ÆŃõ»ÆµĆµ½£¬Ōņ»ÆŗĻĪļ¢õĪŖ“¼Ąą£¬½į¹¹¼ņŹ½£ŗCH2=CH-CH2OH£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ 2CH2=CH-CH2OH+O2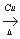 2CH2=CH-CHO+2H2O
2CH2=CH-CHO+2H2O
£Ø4£©»ÆŗĻĪļ¢öŹĒ»ÆŗĻĪļ¢ōµÄĶ¬·ÖŅģ¹¹Ģ壬¢öÄÜÓėNaHCO3ČÜŅŗ·“Ó¦·Å³öĘųĢåµĆµ½¢öŗ¬ōČ»ł£¬ĘäŗĖ“Ź²ÕńĒāĘ×·åĆ껿֮±ČĪŖ1£ŗ1£ŗ6£¬Ōņ»ÆŗĻĪļ¢öµÄ½į¹¹¼ņŹ½ĪŖ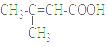
Ąą±Č·“Ó¦¢Ū£¬½įŗĻČ”“ś·“Ó¦µÄĢŲµć¼“æɵƵ½²śĪļµÄ½į¹¹¼ņŹ½”£
”¾Ė¼Ā·µć²¦”æ±¾Ģāæ¼²éĮĖÓŠ»śĪļ¹ŁÄÜĶżäµÄ×Ŗ»Æ£¬±Č½Ļ»ł“””£


 ½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø
½ĢÓżŹĄ¼ŅדŌŖ¾ķĻµĮŠ“š°ø »ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø
»ĘøŌæĪĢĆ×÷Ņµ±¾ĻµĮŠ“š°ø µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
µ„ŌŖ¼ÓĘŚÄ©ø“Ļ°ĻČ·ę“óæ¼¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«×ćĮæµÄCO2²»¶ĻĶØČėNaOH”¢Ba(OH)2”¢Na[Al(OH)4]µÄ»ģŗĻČÜŅŗÖŠ£¬Éś
³É³ĮµķÓėĶØČėCO2µÄĮæµÄ¹ŲĻµæɱķŹ¾ĪŖ
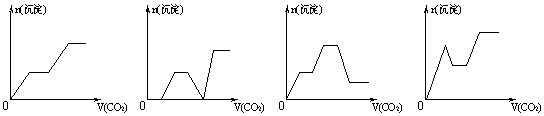 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ(””””)
A£®ĀČĘųµÄŠŌÖŹŗÜ»īĘĆ£¬ĖüÓėĒāĘų»ģŗĻŗóĮ¢¼“·¢Éś±¬ÕØ
B£®æÉŅŌÓĆĒāŃõ»ÆøĘČÜŅŗĪüŹÕŹµŃéŹŅÖĘČ”ĀČĘųŹ±¶ąÓąµÄĀČĘų
C£®¼ģŃéCl2ĘųĢåÖŠŹĒ·ń»ģÓŠHCl·½·ØŹĒ½«ĘųĢåĶØČėĻõĖįŅųČÜŅŗ
D£®³żČ„Cl2ĘųĢåÖŠµÄHC l£¬æɽ«ĘųĢåĶØČė±„
l£¬æɽ«ĘųĢåĶØČė±„ ŗĶŹ³ŃĪĖ®
ŗĶŹ³ŃĪĖ®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀ£¬AŹĒæÉÓĆĄ“¶Ō×ŌĄ“Ė®½ųŠŠĻū¶¾µÄ»ĘĀĢÉ«µ„ÖŹĘųĢ壬A”¢B”¢C”¢D”¢E¶¼ŗ¬XŌŖĖŲ£¬Ęä×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾”£
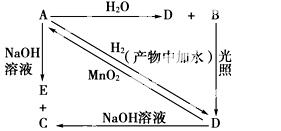
£Ø1£©Ēė·Ö±šŠ“³öA”¢B”¢DµÄ»ÆѧŹ½(ČēĪŖČÜŅŗĒėĢīČÜÖŹµÄ»ÆѧŹ½)£ŗ
A________”¢B________”¢D________£»
£Ø2£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½»ņĄė×Ó·½³ĢŹ½(Ēė×¢Ć÷Ģõ¼ž)£ŗ
A£«H2O(Ąė×Ó·½³ĢŹ½)£ŗ_________________________________________________£»
A£«NaOH(Ąė×Ó·½³ĢŹ½)£ŗ________________________________________________£»
D”śA(»Æѧ·½³ĢŹ½)£ŗ__________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓėŗ¬ĀČ»ÆŗĻĪļÓŠ¹ŲµÄĖµ·ØÕżČ·µÄŹĒ (””””)
A£®HClOŹĒČõĖį£¬ĖłŅŌNaClOŹĒČõµē½āÖŹ
B£®Ļņ·ŠĖ®ÖŠÖšµĪ¼ÓČėÉŁĮ汄ŗĶFeCl3ČÜŅŗ£¬æÉÖʵĆFe(OH)3½ŗĢå
C£®HClČÜŅŗŗĶNaClČÜŅŗ¾łĶعżĄė×Óµ¼µē£¬ĖłŅŌHClŗĶNaCl¾łŹĒĄė×Ó»ÆŗĻĪļ
D£®µē½āNaClČÜŅŗµĆµ½22.4 L H2(±ź×¼×“æö)£¬ĄķĀŪÉĻŠčŅŖ×ŖŅĘNAøöµē×Ó(NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
9.3-ĀČĪģĶéŹĒŅ»ÖÖÓŠ»śŗĻ³ÉÖŠ¼äĢ壬ĻĀĮŠÓŠ¹Ų3-ĀČĪģĶéµÄŠšŹöÕżČ·µÄŹĒ
A.3-ĀČĪģĶéµÄ·Ö×ÓŹ½ĪŖC6H9Cl3 B.3-ĀČĪģĶéŹōÓŚĶéĢž
C.3-ĀČĪģĶéµÄŅ»äå“śĪļ¹²ÓŠ3ÖÖ D.3-ĀČĪģĶéµÄĶ¬·ÖŅģ¹¹Ģå¹²ÓŠ6ÖÖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A£®ŹÆÓĶ·ÖĮó”¢ĆŗµÄĘų»Æ”¢ŗ£Ė®ÖĘŹ³ŃĪ”¢µ°°×ÖŹ±äŠŌµČ¹ż³Ģ¶¼°üŗ¬»Æѧ±ä»Æ
B. ĖįŠŌŃõ»ÆĪļŅ»¶Ø²»ÄÜŗĶĖį·“Ó¦
C. Ļ”¶¹½¬”¢¹čĖį”¢ĀČ»ÆĢśČÜŅŗ¾łĪŖ½ŗĢå
D£®±¬ĆłĘų”¢ĀĮČČ¼Į”¢²£Į§”¢»ØÉśÓĶ”¢¾ŪŅŅĻ©¾łĪŖ»ģŗĻĪļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĮņĖįŌüŹĒÓĆ»ĘĢśæóÖĘŌģĮņĖį¹ż³ĢÖŠÅųöµÄ·ĻŌü£¬Ö÷ŅŖ»Æѧ³É·ÖĪŖSiO2(Ō¼45%)”¢Fe2O3(Ō¼40%)”¢Al2O3(Ō¼10%)ŗĶMgO(Ō¼5%)”£Ä³Ķ¬Ń§Éč¼ĘĮĖČēĻĀ·½°ø£¬·ÖĄėѳʷ֊ø÷ÖÖ½šŹōŌŖĖŲ”£Ēė»Ų“šĻĀĮŠĪŹĢā”£
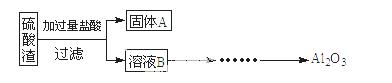
£Ø1£©Š“³öČÜŅŗBµÄČÜÖŹŹĒ ”£
£Ø2£©²ĪÕÕŅŌĻĀæņĶ¼ŠĪŹ½½ųŅ»²½Ķź ³É”°ČÜŅŗC”±µ½”°Al2O3”±µÄĮ÷³Ģ£Ø×¢Ć÷ŹŌ¼Į”¢Ģõ¼žŗĶ²Ł×÷£© ”£
³É”°ČÜŅŗC”±µ½”°Al2O3”±µÄĮ÷³Ģ£Ø×¢Ć÷ŹŌ¼Į”¢Ģõ¼žŗĶ²Ł×÷£© ”£
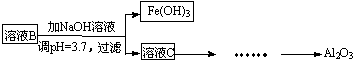
£Ø3£©ĪŖĮĖ·ÖĪöijĮņĖįŌüÖŠĢśŌŖĖŲµÄŗ¬Į棬ĻČ½«ĮņĖįŌüŌ¤“¦Ąķ£¬°ŃĢśŌŖĖŲ»¹Ō³ÉFe2+£¬ŌŁÓĆKMnO4±ź×¼ČÜŅŗŌŚĖįŠŌĢõ¼žĻĀ½ųŠŠŃõ»Æ»¹ŌµĪ¶Ø”£Š“³ö·“Ó¦µÄĄė·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»Æѧъ¾æŠŌѧĻ°Š”×éĪŖĮĖÄ£Äā¹¤ŅµĮ÷³Ģ“ÓÅØĖõµÄŗ£Ė®ÖŠĢįČ”Ņŗä壬²éŌÄ׏ĮĻÖŖ£ŗBr2µÄ·ŠµćĪŖ59”ę£¬Ī¢ČÜÓŚĖ®£¬ÓŠ¶¾ŠŌ”£Éč¼ĘĮĖČēĻĀ²Ł×÷²½Öč¼°Ö÷ŅŖŹµŃé×°ÖĆ£Ø¼Š³Ö×°ÖĆĀŌČ„£©£ŗ
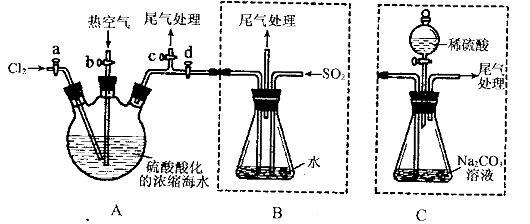
¢ŁĮ¬½ÓAÓėB£¬¹Ų±Õ»īČūb”¢d£¬“ņæŖ»īČūa”¢c£¬ĻņAÖŠ»ŗĀżĶØČėÖĮ·“Ó¦½įŹų£»
¢Ś¹Ų±Õa”¢c£¬“ņæŖb”¢d£¬ĻņAÖŠ¹ÄČė×ćĮæČČæÕĘų£»
¢Ū½ųŠŠ²½Öč¢ŚµÄĶ¬Ź±£¬ĻņBÖŠĶØČė×ćĮæSO2:
¢Ü¹Ų±Õb£¬“ņæŖa£¬ŌŁĶعżAĻņBÖŠ»ŗĀżĶØČė×ćĮæCl2;
¢Ż½«BÖŠĖłµĆŅŗĢå½ųŠŠÕōĮó£¬ŹÕ¼ÆŅŗä唣
Ēė»Ų“š£ŗ
(1)ŹµŃéŹŅÖŠ³£ÓĆĄ“ÖʱøĀČĘųµÄ»Æѧ·½³ĢŹ½ĪŖ__________________________£»
(2)²½Öč¢ŚÖŠ¹ÄČėČČæÕĘųµÄ×÷ÓĆĪŖ_____________________________£»
(3)²½ÖčBÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________”£
(4)“ĖŹµŃéÖŠĪ²ĘųæÉÓĆ £ØĢīŃ”Ļī×ÖÄø£©ĪüŹÕ“¦Ąķ”£
a£®Ė® b£®ÅØĮņĖį c£®NaOHČÜŅŗ d.±„ŗĶNaCIČÜŅŗ
(5)²½Öč¢ŻÖŠ£¬ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÕōĮó£¬ŹÕ¼ÆŅŗä壬½«×°ÖĆĶ¼ÖŠČ±ÉŁµÄ±ŲŅŖŅĒĘ÷²¹»³öĄ“”£
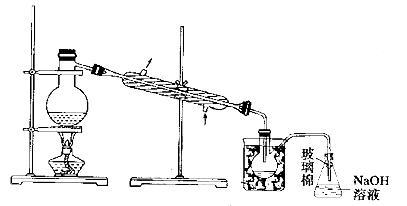
(6)ČōÖ±½ÓĮ¬½ÓAÓėC£¬½ųŠŠ²½Öč¢ŁŗĶ¢Ś£¬³ä·Ö·“Ó¦ŗó£¬Ļņ׶ŠĪĘæÖŠµĪ¼ÓĻ”ĮņĖį£¬ŌŁ¾²½Öč¢Ż£¬Ņ²ÄÜÖʵĆŅŗä唣µĪ¼ÓĻ”ĮņĖįÖ®Ē°£¬CÖŠ·“Ӧɜ³ÉĮĖNaBrO3µČ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________”£
(7)ÓėB×°ÖĆĻą±Č£¬²ÉÓĆC×°ÖƵÄÓŵćĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com